Rotator Cuff Tears: Pathology and Repair
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Rotator Cuff and Subacromial Impingement Syndrome: Anatomy, Etiology, Screening, and Treatment
Rotator Cuff and Subacromial Impingement Syndrome: Anatomy, Etiology, Screening, and Treatment The glenohumeral joint is the most mobile joint in the human body, but this same characteristic also makes it the least stable joint.1-3 The rotator cuff is a group of muscles that are important in supporting the glenohumeral joint, essential in almost every type of shoulder movement.4 These muscles maintain dynamic joint stability which not only avoids mechanical obstruction but also increases the functional range of motion at the joint.1,2 However, dysfunction of these stabilizers often leads to a complex pattern of degeneration, rotator cuff tear arthropathy that often involves subacromial impingement.2,22 Rotator cuff tear arthropathy is strikingly prevalent and is the most common cause of shoulder pain and dysfunction.3,4 It appears to be age-dependent, affecting 9.7% of patients aged 20 years and younger and increasing to 62% of patients of 80 years and older ( P < .001); odds ratio, 15; 95% CI, 9.6-24; P < .001.4 Etiology for rotator cuff pathology varies but rotator cuff tears and tendinopathy are most common in athletes and the elderly.12 It can be the result of a traumatic event or activity-based deterioration such as from excessive use of arms overhead, but some argue that deterioration of these stabilizers is part of the natural aging process given the trend of increased deterioration even in individuals who do not regularly perform overhead activities.2,4 The factors affecting the rotator cuff and subsequent treatment are wide-ranging. The major objectives of this exposition are to describe rotator cuff anatomy, biomechanics, and subacromial impingement; expound upon diagnosis and assessment; and discuss surgical and conservative interventions. -

About Soft Tissue Sarcoma Overview and Types
cancer.org | 1.800.227.2345 About Soft Tissue Sarcoma Overview and Types If you've been diagnosed with soft tissue sarcoma or are worried about it, you likely have a lot of questions. Learning some basics is a good place to start. ● What Is a Soft Tissue Sarcoma? Research and Statistics See the latest estimates for new cases of soft tissue sarcoma and deaths in the US and what research is currently being done. ● Key Statistics for Soft Tissue Sarcomas ● What's New in Soft Tissue Sarcoma Research? What Is a Soft Tissue Sarcoma? Cancer starts when cells start to grow out of control. Cells in nearly any part of the body can become cancer and can spread to other areas. To learn more about how cancers start and spread, see What Is Cancer?1 There are many types of soft tissue tumors, and not all of them are cancerous. Many benign tumors are found in soft tissues. The word benign means they're not cancer. These tumors can't spread to other parts of the body. Some soft tissue tumors behave 1 ____________________________________________________________________________________American Cancer Society cancer.org | 1.800.227.2345 in ways between a cancer and a non-cancer. These are called intermediate soft tissue tumors. When the word sarcoma is part of the name of a disease, it means the tumor is malignant (cancer).A sarcoma is a type of cancer that starts in tissues like bone or muscle. Bone and soft tissue sarcomas are the main types of sarcoma. Soft tissue sarcomas can develop in soft tissues like fat, muscle, nerves, fibrous tissues, blood vessels, or deep skin tissues. -

Bilateral Simultaneous Rupture of the Quadriceps Tendons in Healthy Individuals Takuro Moriya1,2* and Abe Yoshihiro1
Moriya et al. Trauma Cases Rev 2016, 2:043 Volume 2 | Issue 3 ISSN: 2469-5777 Trauma Cases and Reviews Case Report: Open Access Bilateral Simultaneous Rupture of the Quadriceps Tendons in Healthy Individuals Takuro Moriya1,2* and Abe Yoshihiro1 1Department of Orthopaedic Surgery, Chiba Rosai Hospital, Japan 2Department of Orthopaedic Surgery, Chiba Kaihin Municipal Hospital, Japan *Corresponding author: Takuro Moriya, Department of Orthopaedic Surgery, Chiba Rosai Hospital, 2-16 Tatsumidai- higashi, Ichihara 290-0003, Japan, Tel: +81-436-74-1111, Fax: +81-436-74-1151, E-mail: [email protected] Abstract Quadriceps tendon rupture is an uncommon injury in healthy individuals. This paper presents two case reports of patients of bilateral quadriceps tendon rupture, who were misdiagnosed as muscle weakness of quadriceps with contusion of the knee joint. Subsequent physical examination showed a supra-patellar gap, moderate hemarthrosis of both knees, and failure of active knee extension. MRI showed bilateral rupture of the quadriceps tendons at the osteotendinous junction. Radiographs described the depression in the suprapatellar soft tissue, patella baja and an avulsion bony fragment on the patella. Surgery confirmed the MRI observation, so transosseous suturing and augmentation were undertaken. Both patients returned to a normal life with useful function. Adequate physical examination and correct understanding of both radiograph and MRI were required to prevent misdiagnosis. Keywords Bilateral quadriceps tendon ruptures, Healthy individuals Figure 1: Suprapatellar gap in Case 1. Introduction avulsion fragment on the patella (Figure 3). The patellar heights of his right and left knees were 0.89 and 0.68, respectively, using the Insall- Rupture of the quadriceps tendon is an uncommon injury, and Saivati method. -

Musculoskeletal Diagnostic Imaging
Musculoskeletal Diagnostic Imaging Vivek Kalia, MD MPH October 02, 2019 Course: Sports Medicine for the Primary Care Physician Department of Radiology University of Michigan @VivekKaliaMD [email protected] Objectives • To review anatomy of joints which commonly present for evaluation in the primary care setting • To review basic clinical features of particular musculoskeletal conditions affecting these joints • To review key imaging features of particular musculoskeletal conditions affecting these joints Outline • Joints – Shoulder – Hip • Rotator Cuff Tendinosis / • Osteoarthritis Tendinitis • (Greater) Trochanteric bursitis • Rotator Cuff Tears • Hip Abductor (Gluteal Tendon) • Adhesive Capsulitis (Frozen Tears Shoulder) • Hamstrings Tendinosis / Tears – Elbow – Knee • Lateral Epicondylitis • Osteoarthritis • Medical Epicondylitis • Popliteal / Baker’s cyst – Hand/Wrist • Meniscus Tear • Rheumatoid Arthritis • Ligament Tear • Osteoarthritis • Cartilage Wear Outline • Joints – Ankle/Foot • Osteoarthritis • Plantar Fasciitis • Spine – Degenerative Disc Disease – Wedge Compression Deformity / Fracture Shoulder Shoulder Rotator Cuff Tendinosis / Tendinitis • Rotator cuff comprised of 4 muscles/tendons: – Supraspinatus – Infraspinatus – Teres minor – Subscapularis • Theory of rotator cuff degeneration / tearing with time: – Degenerative partial-thickness tears allow superior migration of the humeral head in turn causes abrasion of the rotator cuff tendons against the undersurface of the acromion full-thickness tears may progress to -

Primary and Secondary Consequences of Rotator Cuff
Hafizur Rahman Department of Mechanical Science and Engineering, University of Illinois at Urbana-Champaign, Urbana, IL 61801 Primary and Secondary e-mail: [email protected] Consequences of Rotator Cuff Eric Currier Department of Mechanical Science and Engineering, Injury on Joint Stabilizing University of Illinois at Urbana-Champaign, Urbana, IL 61801 Tissues in the Shoulder e-mail: [email protected] Rotator cuff tears (RCTs) are one of the primary causes of shoulder pain and dysfunction Marshall Johnson in the upper extremity accounting over 4.5 million physician visits per year with 250,000 Department of Mechanical Engineering, rotator cuff repairs being performed annually in the U.S. While the tear is often consid- Georgia Institute of Technology, ered an injury to a specific tendon/tendons and consequently treated as such, there are Atlanta, GA 30332 secondary effects of RCTs that may have significant consequences for shoulder function. e-mail: [email protected] Specifically, RCTs have been shown to affect the joint cartilage, bone, the ligaments, as well as the remaining intact tendons of the shoulder joint. Injuries associated with the Rick Goding upper extremities account for the largest percent of workplace injuries. Unfortunately, Department of Orthopaedic, the variable success rate related to RCTs motivates the need for a better understanding Joint Preservation Institute of Iowa, of the biomechanical consequences associated with the shoulder injuries. Understanding West Des Moines, IA 50266 the timing of the injury and the secondary anatomic consequences that are likely to have e-mail: [email protected] occurred are also of great importance in treatment planning because the approach to the treatment algorithm is influenced by the functional and anatomic state of the rotator cuff Amy Wagoner Johnson and the shoulder complex in general. -

Impingement Syndrome and Tears of the Rotator Cuff
Impingement Syndrome and Tears of the Rotator Cuff Dr Keith Holt Impingement is a very common problem in which the tendons of the rotator cuff (predominantly supraspinatus) rub on the underside of the acromion (the bone at the point of the shoulder). This causes pain due to the repeated rubbing of those tendons and it is especially bad with certain positions of the arm. In particular it is difficult to put the arm behind the back and to use it in the elevated position. This makes it difficult to drive, change gears, hang clothes, comb one’s hair, and even to lie on the affected shoulder. The cause of this problem can be: How does the shoulder work? The shoulder, like the hip, is a ball and socket joint (like a tow 1) A muscle imbalance problem due to poor functioning bar). Unlike the hip however, the socket is very small and is of the rotator cuff tendons themselves; thus allowing the not big enough to hold the head of the humerus (the ball) arm to ride up and rub on the acromion, squashing the in place. This gives the joint a large range of motion but, as rotator cuff tendons in the process: or a consequence, it also means that it is potentially unstable. 2) A mechanical problem where the space for the tendon To function normally, muscles on both sides of the joint must is inadequate. One way this can occur is with an injury work together to hold the joint in place during movement. to the tendon itself which causes swelling of that tendon This means that when the deltoid muscle (see diagrams) lifts such that it becomes too large for the space at hand the arm out from the side of the body, the supraspinatus and [primary tendonitis (inflammation of the tendon) with other muscles of the rotator cuff must pull down on the top secondary impingement [rubbing of the tendon on the of the humerus. -

Rotator Cuff Tears
OrthoInfo Basics Rotator Cuff Tears What is a rotator cuff? One of the Your rotator cuff helps you lift your arm, rotate it, and reach up over your head. most common middle-age It is made up of muscles and tendons in your shoulder. These struc- tures cover the head of your upper arm bone (humerus). This “cuff” complaints is holds the upper arm bone in the shoulder socket. shoulder pain. Rotator cuff tears come in all shapes and sizes. They typically occur A frequent in the tendon. source of that Partial tears. Many tears do not completely sever the soft tissue. Full thickness tears. A full or "complete" tear will split the soft pain is a torn tissue into two, sometimes detaching the tendon from the bone. rotator cuff. Rotator Cuff Bursa A torn rotator cuff will Tendon Clavicle (Collarbone) Humerus weaken your shoulder. (Upper Arm) This means that many Normal shoulder anatomy. daily activities, like combing your hair or Scapula getting dressed, may (Shoulder Blade) become painful and difficult to do. Rotator Cuff Tendon A complete tear of the rotator cuff tendon. 1 OrthoInfo Basics — Rotator Cuff Tears What causes rotator cuff tears? There are two main causes of rotator cuff repeating the same shoulder motions again and tears: injury and wear. again. Injury. If you fall down on your outstretched This explains why rotator cuff tears are most arm or lift something too heavy with a jerking common in people over 40 who participate in motion, you could tear your rotator cuff. This activities that have repetitive overhead type of tear can occur with other shoulder motions. -

Open Fracture As a Rare Complication of Olecranon Enthesophyte in a Patient with Gout Rafid Kakel, MD, and Joseph Tumilty, MD
A Case Report & Literature Review Open Fracture as a Rare Complication of Olecranon Enthesophyte in a Patient With Gout Rafid Kakel, MD, and Joseph Tumilty, MD has been reported in the English literature. The patient Abstract provided written informed consent for print and elec- Enthesophytes are analogous to osteophytes of osteo- tronic publication of this case report. arthritis. Enthesopathy is the pathologic change of the enthesis, the insertion site of tendons, ligaments, and CASE REPORT joint capsules on the bone. In gout, the crystals of mono- A 50-year-old man with chronic gout being treated with sodium urate monohydrate may provoke an inflamma- tory reaction that eventually may lead to ossification at allopurinol (and indomethacin on an as-needed basis) those sites (enthesophytes). Here we report the case of presented to the emergency department. He reported a man with chronic gout who sustained an open fracture of an olecranon enthesophyte when he fell on his left elbow. To our knowledge, no other case of open fracture of an enthesophyte has been reported in the English literature. nthesophytes are analogous to osteophytes of osteoarthritis. Enthesopathy is the pathologic change of the enthesis, the insertion site of ten- dons, ligaments, and joint capsules on the bone. EEnthesopathy occurs in a wide range of conditions, notably spondyloarthritides, crystal-induced diseases, and repeated minor trauma to the tendinous attach- ments to bones. Enthesopathy can be asymptomatic or symptomatic. In gout, crystals of monosodium urate are found in and around the joints—the cartilage, epiphyses, synovial membrane, tendons, ligaments, and enthesis. Figure 1. Small wound at patient’s left elbow. -
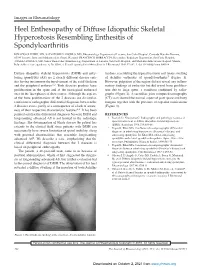
Heel Enthesopathy of Diffuse Idiopathic Skeletal Hyperostosis
Images in Rheumatology Heel Enthesopathy of Diffuse Idiopathic Skeletal Hyperostosis Resembling Enthesitis of Spondyloarthritis IGNAZIO OLIVIERI, MD, SALVATORE D’ANGELO, MD, Rheumatology Department of Lucania, San Carlo Hospital, Contrada Macchia Romana, 85100 Potenza, Italy; and Madonna delle Grazie Hospital; FRANCESCO BORRACCIA, Researcher, Radiology Department, San Carlo Hospital; ANGELA PADULA, MD, Senior Researcher, Rheumatology Department of Lucania, San Carlo Hospital, and Madonna delle Grazie Hospital, Matera, Italy. Address correspondence to Dr. Olivieri; E-mail: [email protected]. J Rheumatol 2010;37:192–3; doi.10.3899/jrheum.090514 Diffuse idiopathic skeletal hyperostosis (DISH) and anky- tendons, resembling the typical fusiform soft tissue swelling losing spondylitis (AS) are 2 clearly different disease enti- of Achilles enthesitis of spondyloarthritis5 (Figure 1). ties having in common the involvement of the axial skeleton However, palpation of the region did not reveal any inflam- and the peripheral entheses1,2. Both diseases produce bone matory findings of enthesitis but did reveal bone prolifera- proliferation in the spine and at the extraspinal entheseal tion due to large spurs, a condition confirmed by radio- sites in the later phases of their course. Although the aspects graphs (Figure 2). A sacroiliac joint computed tomography of the bone proliferations of the 2 diseases are dissimilar, (CT) scan showed the normal aspect of joint space and bony confusion of radiographic differential diagnosis between the margins together with the presence of capsular ossifications 2 diseases exists, partly as a consequence of a lack of aware- (Figure 3). ness of their respective characteristic features2,3. It has been pointed out that the differential diagnosis between DISH and REFERENCES longstanding advanced AS is not limited to the radiologic 1. -
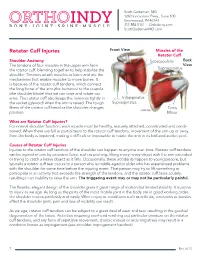
Rotator Cuff Injuries
Scott Gudeman, MD 1260 Innovation Pkwy., Suite 100 Greenwood, IN 46143 317.884.5161 OrthoIndy.com ScottGudemanMD.com Rotator Cuff Injuries Front View Muscles of the Rotator Cuff Shoulder Anatomy Subscapularis Back The tendons of four muscles in the upper arm form View Supraspinatus the rotator cuff, blending together to help stabilize the shoulder. Tendons attach muscles to bone and are the mechanisms that enable muscles to move bones. It is because of the rotator cuff tendons, which connect the long bone of the arm (the humerus) to the scapula (the shoulder blade) that we can raise and rotate our arms. The rotator cuff also keeps the humerus tightly in Infraspinatus the socket (glenoid) when the arm is raised. The tough Supraspinatus fibers of the rotator cuff bend as the shoulder changes Teres position. Minor What are Rotator Cuff Injuries? For normal shoulder function, each muscle must be healthy, securely attached, coordinated and condi- tioned. When there are full or partial tears to the rotator cuff tendons, movement of the arm up or away from the body is impaired, making it difficult or impossible to rotate the arm in its ball-and-socket joint. Causes of Rotator Cuff Injuries Injuries to the rotator cuff tendons of the shoulder can happen to anyone over time. Rotator cuff tendons can be injured or torn by excessive force, such as pitching, lifting a very heavy object with the arm extended or trying to catch a heavy object as it falls. Occasionally, these accidents happen to young people, but typically a rotator cuff tear occurs to a person who is middle-aged or older who has experienced problems with the shoulder for some time before the injuring event. -

Acute Management of Soft Tissue Injuries
www.acpsm.org Acute Management of Soft Tissue Injuries Protection, Rest, Ice, Compression, and Elevation Guidelines www.acpsm.org Management of acute soft tissue injury using Protection Rest Ice Compression and Elevation: Recommendations from the Association of Chartered Physiotherapists in Sports and Exercise Medicine (ACPSM) Chris M Bleakley1, Philip D Glasgow2, Nicola Phillips2, Laura Hanna2, Michael J Callaghan2, Gareth W Davison3, Ty J Hopkins3, Eamonn Delahunt3 1Lead author and guarantor 2ACPSM consensus panel 3External authors and contributors Acknowledgements to other ACPSM contributors: Lynn Booth, Nicola Combarro, Sian Knott, Chris McNicholl and Colin Paterson for their assistance with literature searching, data extraction, and interpretation of outcomes. This work was funded by the Association of Chartered Physiotherapists in Sports and Exercise Medicine (ACPSM). The guidelines are endorsed by the Chartered Society of Physiotherapy’s Supporting Knowledge in Physiotherapy Practice Programme (SKIPP), after peer review from their Good Practice Panel in October 2010. CONTENTS Chapter 1: Project methods Chapter 2: What is the magnitude and depth of cooling associated with ice? Chapter 3: Can PRICE decrease the infl ammatory response after acute soft tissue injury? Chapter 4: What effect does mechanical loading have on infl ammation and soft tissue healing after acute injury? Chapter 5: Do the physiological effects of local tissue cooling affect function, sporting performance and injury risk? Chapter 6: Which components of PRICE are effective in the clinical management of acute soft tissue injury? Chapter 7: Executive summary Chapter 8: Appendices Chapter 1 Project methods Background The need for guidelines Soft tissue injury is a common problem in sport, recreational and physical activities. -

Rotator Cuff Tendinitis Shoulder Joint Replacement Mallet Finger Low
We would like to thank you for choosing Campbell Clinic to care for you or your family member during this time. We believe that one of the best ways to ensure quality care and minimize reoccurrences is through educating our patients on their injuries or diseases. Based on the information obtained from today's visit and the course of treatment your physician has discussed with you, the following educational materials are recommended for additional information: Shoulder, Arm, & Elbow Hand & Wrist Spine & Neck Fractures Tears & Injuries Fractures Diseases & Syndromes Fractures & Other Injuries Diseases & Syndromes Adult Forearm Biceps Tear Distal Radius Carpal Tunnel Syndrome Cervical Fracture Chordoma Children Forearm Rotator Cuff Tear Finger Compartment Syndrome Thoracic & Lumbar Spine Lumbar Spine Stenosis Clavicle Shoulder Joint Tear Hand Arthritis of Hand Osteoporosis & Spinal Fx Congenital Scoliosis Distal Humerus Burners & Stingers Scaphoid Fx of Wrist Dupuytren's Comtracture Spondylolysis Congenital Torticollis Shoulder Blade Elbow Dislocation Thumb Arthritis of Wrist Spondylolisthesis Kyphosis of the Spine Adult Elbow Erb's Palsy Sprains, Strains & Other Injuries Kienböck's Disease Lumbar Disk Herniation Scoliosis Children Elbow Shoulder Dislocation Sprained Thumb Ganglion Cyst of the Wrist Neck Sprain Scoliosis in Children Diseases & Syndromes Surgical Treatments Wrist Sprains Arthritis of Thumb Herniated Disk Pack Pain in Children Compartment Syndrome Total Shoulder Replacement Fingertip Injuries Boutonnière Deformity Treatment