Biobed Rotation to Vertical
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Flyer2021bibliobusweb-44104.Pdf
Bibliothèques Comment municipales une fenêtre sur le monde nous joindre ? la réponse est humaine Par téléphone : 022 418 92 70 Vous avez une question et vous souhaitez (répondeur 24h/24h, laissez votre nom une réponse personnalisée ? InterroGE est et numéro d’emprunteur) un service de renseignements à distance accessible par formulaire. Le réseau des Par e-mail : hors-murs.bmu @ville-ge.ch bibliothèques genevoises vous offre, en bibli-o-bus Toutes les informations sur : www.bm-geneve.ch moins de trois jours, un résultat fiable et des sources identifiées. horaires www.interroge.ch & infos pratiques janvier à décembre 2021 s’inscrire L’emprunt de documents à domicile ainsi que l’accès aux ressources numériques à distance nécessitent une inscription préalable au Le bibli-o-bus bibli-o-bus ou dans l’une des bibliothèques municipales de la ville de Genève (voir condi- LE BIBLI-O-BUS EST UN SERVICE PROPOSÉ PAR LA VILLE tions sur notre site internet). DE GENÈVE EN COLLABORATION AVEC L’ASSOCIATION DES emprunter COMMUNES GENEVOISES. QUATRE VÉHICULES DESSERVENT Empruntez jusqu’à 20 documents et 10 magazines sur l’ensemble du réseau 28 COMMUNES DANS 34 POINTS DE STATIONNEMENT. pour une période de 28 jours. Vous y trouverez un vaste choix de documents pour adultes, enfants et adolescent-e s : des romans, des documentaires, des livres lus ou en prolonger Prolongez jusqu’à 3 fois vos emprunts auprès grands caractères, des bandes dessinées, des albums illustrés, des bibliothécaires, par téléphone, par e-mail des films et bien plus encore. ou via votre espace personnel en ligne. -

Dialogue Winter 2019/20 | 1 Editorial (Continued)
the Banque Cantonale de Genève magazine | Winter 2019 / 20 Best practices and added value for companies Private asset management accessible from CHF 50,000 thanks to Best of Genesis BCGE L’essentiel de la finance The dynamic municipality of Presinge continues the renovation of its historic buildings 051219 1816/CH/E/ Simple Secure Smart Online trading: take control! Do you prefer to manage your stock market investments independently? Take the bull by the horns and trade using a site that gives you everything you need: ✔ brokerage fees from CHF 8 ✔ management of accounts and payments on an integrated platform ✔ access to all the financial information Request for access: ■ email at [email protected] ■ ask a relationship manager at Banque Cantonale de Genève ■ call 058 211 21 00 1816 BCGE.ch 1816-CH-E-051219-APPROVED-210x297-BCGE-1816-19BCGE417.indd 1 06.12.19 09:52 1816-CH-E-051219-APPROVED-210x297-BCGE-1816-19BCGE417.indd 5 décembre, 2019 Editorial Contents Active discretionary Economic outlook Geneva economic dashboard 2 management: What are the implications for investments in an environment a resilient species of negative interest rates and a strong Swiss franc? 4 Blaise Goetschin Sustainable development CEO Best practices and added value for companies 5 BCGE visits the UN 6 Darwinism, the theory of competitive species selection, is also widespread in the financial SME QR-bill: get ready now 6 world. The methods used to manage securities portfolios are directly concerned. Indeed, the Investment Private asset management accessible from CHF 50,000 framework conditions for asset management have thanks to Best of Genesis 7 been undergoing profound structural changes The Synchrony LPP 80 B embodies a more dynamic in recent years: technological developments, and less cautious pension planning 8 artificial intelligence and robotisation, regulatory Analysis – L’essentiel de la finance developments, standardisation of advisory processes Recession or no recession? 9 and investor protection. -
F R a N C E F R a N C E F R a N
33 Bellebouche 33 Bellebouche Les Mermes La Place Les Pavillons Corsier t n a n r e u T y r é N v o C e FRANCE d e d C h e m in u a e e t s Les Étoles Foncenex s u Ch i em u o in de R R 31 n de éo -L s Bellebouche -à e e Bois - Brûlés r n è R i o ig FRANCE n ut V e g y i T uernant r C la a é h. t e â v d o la h . B C h C ts e Bois de C e a d u s r Rou d e p te e e h des r Rui d Merlinge d C ss e é eau Ét u oile o s i l r y a de V G e B s B d ra Prés de e el Gar si d R l e s è ui b m r e ss s o a e e ea s u i d d Villette u L li e c se 32 e u d h o e N F r ant u e ne t . ig u R s e o e e -S 30 R d t . u . u h s d h Gy - Les Creuses e o C s C d t R e e . n h h b g A Gy-Temple m C C i a V h s 33 C e s ll Les Trembles t o s de 34 t e R r t É d o u he e u t e C b o m i l s a s r e 25 h e R a d 26 d e V . -

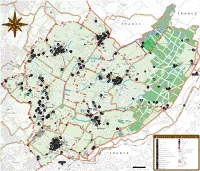
Les Chemins Historiques Du Canton De Genève
Carte de terrain IVS Carte de terrain Talus et délimitation Matériau meuble Rocher Mur de soutènement Mur, mur de parapet Alignement d’arbres, haie Pierre bordière, bordure Dalles bordières verticales Clôture, palissade Revêtements Les chemins historiques Rocher Matériau meuble Empierrement, cailloutis du canton de Genève Pavage, pavement Revêtement artificiel Marches, escaliers, gradins Ouvrages d’art Pont Vestiges de pont Conduite d’eau, tombino Carte d’inventaire IVS Tunnel GE Eléments du paysage routier Pierre de distance Autre pierre Arbre isolé Inscription Croix routière Oratoire, chapelle routière Chapelle Eglise Château-fort, château, ruine Edifice profane Exploitation industrielle Carrière, gravière Embarcadère, port Fontaine Carte d’inventaire Classification Importance nationale Importance régionale Importance locale Substance Tracé historique Tracé historique avec substance Tracé historique avec beaucoup de substance Inventar historischer Verkehrswege der Schweiz Inventaire des voies de communication historiques de la Suisse Inventario delle vie di comunicazione storiche della Svizzera Inventari da las vias da communicaziun istoricas da la Svizra Les chemins historiques d’importance nationale dans le canton de Genève Numération selon l’IVS 1 Genève–Nyon (–Lausanne) 2 Genève–frontière nationale (–Fort-de-L’Ecluse, F) 3 Genève–Carouge–St-Julien-en- Page de couverture Sources des illustrations Nyon Genevois, F (–Seyssel, F) Diversité des chemins historiques dans le Les photos du terrain sont de Yves Bischof- 4 Genève–Carouge–Croix-de-Rozon canton de Genève: chemin creux d’origine berger. Les autres sources sont mentionnées (–Annecy, F) romaine dans une propriété privée à Frontenex dans les légendes. Reproduction des extraits 5 Genève–Veyrier (–Pas-de-l’Echelle, F (à gauche; GE 6.2.1; cf. -

Communes\Communes - Renseignements 2019.07.Xlsx 02.07.19
TAXE PROFESSIONNELLE COMMUNALE Contacts par commune No COMMUNE PERSONNE(S) DE CONTACT No Tél No Fax E-mail 1 Aire-la-Ville Véronique DUPERRIER 022 757 48 29 022 757 48 32 [email protected] 2 Anières Dominique LAZZARELLI 022 751 83 05 022 751 28 61 [email protected] 3 Avully Véronique SCHMUTZ 022 756 92 50 022 756 92 59 [email protected] 4 Avusy Alfred MAGNIN 079 686 85 18 022 756 90 61 [email protected] 5 Bardonnex Marie-Laurence MICHAUD-GAUD 022 721 02 20 022 721 02 29 [email protected] 6 Bellevue Pierre DE GRIMM 022 959 84 34 022 959 88 21 [email protected] 7 Bernex Nelly BARTHOULOT 022 850 92 92 022 850 92 83 [email protected] 8 Carouge P. GIUGNI / P.CARCELLER 022 307 89 89 022 307 89 39 [email protected] 9 Cartigny Patric HESS 022 756 12 77 022 756 30 93 [email protected] 10 Céligny Véronique SCHMUTZ 022 776 21 26 022 776 71 55 [email protected] 11 Chancy Alfred MAGNIN 079 686 85 18 - [email protected] 12 Chêne-Bougeries Gwenaële CURCIO 022 869 17 17 - [email protected] 13 Chêne-Bourg Sandra Garcia BAYERL 022 869 41 21 022 348 15 80 [email protected] 14 Choulex Anne-Françoise MOREL 022 707 44 61 - [email protected] 15 Collex-Bossy Yvan MASSEREY 022 959 77 00 - [email protected] 16 Collonge-Bellerive Francisco CHAPARRO 022 722 11 50 022 722 11 66 [email protected] 17 Cologny Daniel WYDLER 022 737 49 51 022 737 49 50 [email protected] 18 Confignon Soheila KHAGHANI 022 850 93 76 022 850 93 92 [email protected] 19 Corsier Raphaël CONTI 022 751 90 60 022 751 90 69 [email protected] 20 Dardagny -

Mobility Plans a Guide for Companies and Institutions May 2016 May
MOBILITY PLANS A GUIDE FOR COMPANIES AND INSTITUTIONS MAY 2016 MAY CONTENTS EDITORIAL 4 – 5 THE LAKE GENEVA METROPOLIS, A DYNAMIC REGION 6 – 7 A COMPANY MOBILITY PLAN 9 – 12 GOOD PRACTICES 13 – 18 STAGES IN THE IMPLEMENTATION OF A MOBILITY PLAN 19 FACTORS FOR SUCCESS 20 EXAMPLES OF COMPANIES WHO HAVE ALREADY ADOPTED AN ACTIVE APPROACH TO MOBILITY MANAGEMENT 21 – 22 TESTIMONIES 23 – 45 N°1 SIG (SERVICES INDUSTRIELS DE GENÈVE) 24 – 25 N°2 GENEVA AIRPORT 26 – 27 N°3 COVANCE 28 – 29 N°4 ZIPLO (ZONE INDUSTRIELLE DE PLAN-LES-OUATES) 30 – 31 N°5 ICRC (INTERNATIONAL COMMITTEE OF THE RED CROSS) 32 – 33 N°6 IMAD (GENEVA INSTITUTE FOR HOMECARE SERVICES) 34 – 35 N°7 LE GROUPEMENT HOSPITALIER DE L’OUEST LÉMANIQUE 36 – 37 N°8 NESTLÉ SUISSE SA IN ORBE 38 – 39 N°9 RETRAITES POPULAIRES 40 – 41 N°10 DANIEL WILLI S.A. 42 – 43 N°11 DENTSPLY MAILLEFER 44 – 45 TO FIND OUT MORE 46 GUIDE TO MOBILITY PLANS / 3 EDITORIAL COMPLEMENTARY MODES OF TRANSPORT, COMPLEMENTARY ACTIONS FOR SUSTAINABLE MOBILITY The Lake Geneva metropolis owes a share of its tremendous economic drive and enviable quality of life to its accessibility. Its excellent rail and road networks, and the presence of an international airport on its territory, guarantee the mobility of the people, information and goods essential for long-term competitiveness today. Though leisure is becoming an increasingly signifi cant aspect of travel requirements, commuting still represents over 50% of transport fl ows. In the face of increasing road congestion, the development of sustainable infrastructures by public authorities (train, public transport and bicycle connections) cannot alone meet employee needs. -

RÉSIDENCES DE LA LOUVIÈRE 9 APPARTEMENTS EN PPE À PRESINGE – GE Ouvrage 2489
Version online sur la plateforme www.architectes.ch RÉSIDENCES DE LA LOUVIÈRE 9 APPARTEMENTS EN PPE À PRESINGE – GE ouvrage 2489 Maître de l’ouvrage Effel Développement SA Rue du Mont-Blanc 14 1201 Genève Architecte Elemento Architecture SA Ch. Ph-De-Sauvage 37 1219 Châtelaine Direction des travaux : Alain Dreier Bureau Technique du Bâtiment SA Place de l’Eglise 2 1232 Confignon Entreprise générale Immolac Engineering SA Rue Général Boinod 20 1170 Aubonne Chef de projet : François Legrand Ingénieur civil Thomas Jundt Ingénieurs civils SA Rue de la Fontenette 27 1227 Carouge Bureaux techniques Chauffage – Géotechnique : Bosson et Pillet SA Avenue des Morgines 23 1213 Petit-Lancy Ventilation : Thiébauld + Perritaz SA Route de Pré-Marais 20 SITUATION Bordant la zone agricole, dans un contexte rural, la PPE a 1233 Bernex été intégrée dans le vaste parc arborisé d’une ancienne Sanitaire : Une petite commune de 700 habitants. La croissance propriété. Une vieille bâtisse qui abrite un EMS jouxte le Claude Burtin, de Presinge s’est faite à partir de quelques mas isolés projet. Les appartements se trouvent au calme, dans un Réalisation Sanitaire sans aboutir à un bâti historiquement cohérent ni un environnement paysager, et sont entourés d’un couronne Rue de la Servette 19 centre bien défini. Le développement récent s’est effec- arborisée. 1203 Genève tué selon les règlements constructifs applicables et la lo- Electricité : gique de la desserte automobile. Pourtant, tous les lieux Egg-Telsa SA et équipements traditionnels de l’identité d’un village, PROGRAMME Rue Guillaume-De-Marcossay 14 mairie, école, église, restaurant et salle communale, sont 1205 Genève présents dans un périmètre restreint. -

Geneva : 45 Communes
Geneva : 45 communes al Aire-la-Ville co Gy am Anières cp Hermance an Avully cq Jussy ao Avusy cr Laconnex ap Bardonnex cs Ville de Lancy aq Bellevue ct Meinier ar Bernex dk Meyrin as Ville de Carouge dl Onex at Cartigny dm Perly Certoux bk Céligny dn Plan-les-Ouates bl Chancy do Pregny Chambesy bm Chêne-Bougeries dp Presinge bn Chêne-Bourg dq Puplinge bo Choulex dr Russin bp Collex-Bossy ds Satigny bq Collonge-Bellerive dt Soral br Cologny ek Thônex bs Confi gnon el Troinex bt Corsier em Vandoeuvres ck Dardagny en Vernier cl Genève eo Versoix cm Genthod ep Veyrier cn Grand-Saconnex IF YOU HAVE DIFFICULTY READING THESE TEXTS, A LARGE FORMAT (A4) VERSION IS AVAILABLE ON : www.ge.ch/integration/publications OR BY CONTACTING THE OFFICE FOR INTEGRATION OF FOREIGNERS, al RUE PIERRE FATIO 15 (4th FLOOR) 1204 GENEVA TEL. 022 546 74 99 www.ge.ch/integration [email protected] 2 WELCOME TO GENEVA - MESSAGE On behalf of the Council of State of the Canton of Geneva and the Association of Geneva Communes (ACG), we extend you a hearty welcome. Geneva has been a place of asylum and refuge for victims of religious persecution since the 16th century, with over a third of its population made up of foreigners. Diversity remains one of the major strengths of Geneva society, in which 194 nationalities are represented today, and solidarity and respect for cultural differences are among the priorities of the political and administrative authorities of both the Canton and the communes of Geneva. -

Map of Fare Zone
Fares Public transport for Geneva Map of Fare Zone as of Dec. 15 2019 Évian-les-Bains Plan tarifaire 300 L1 Thonon-les-Bains Légende Legend Toward Lausanne LignesTrain lines ferroviaires Lac Léman Perrignier Coppet LignesBus and de tram bus etlines tram Chens-sur-Léman LignesTransalis Transalis lines L1 L2 L3 L4 RE Gex Tannay LignesLacustre navettes shuttle lacustre lines Hermance-Village Customs Veigy-Foncenex, Les Cabrettes Bons-en-Chablais Divonne-les-Bains Mies PassageZone crossing de zone Chavannes-des-Bois Hermance Veigy-Foncenex ZoneLéman Léman Pass Pass zones Veigy- Veigy-Village Bois-Chatton Versoix Zoneunireso 10 zoneunireso 10 Pont-Céard Douane 200 Machilly Collex-Bossy Versoix Anières Customs Gy 250 Customs Bossy Genthod Creux-de-Genthod Anières-Douane Corsier Meinier Jussy St-Genis-Pouilly Ferney-Douane Genthod-Bellevue Bellevue Customs Grand-Saconnex-Douane Collonge-Bellerive Ferney-Bois Candide Les Tuileries Customs Le Grand-Saconnex Mategnin Chambésy Choulex Meyrin-Gravière Pregny- Chambésy 10 Genève-Aéroport Genève-Sécheron Vésenaz Presinge Customs Meyrin L1 Saint-Genis-Porte de France L2 Vandœuvre Meyrin L3 CERN 10 De-Chateaubriand Puplinge Thoiry Vernier L4 RE Ville-la-Grand Vernier Gare de Genève Pâquis Port-Noir Zimeysa Chêne-Bourg Annemasse Satigny Ambilly Eaux-Vives Chêne-Bougeries Genève Annemasse Satigny Gaillard- Molard Chêne-Bourg Customs Libération L1 L2 L3 L4 RE Etrembières Le Rhône 10 Genève-Eaux-Vives Moillesulaz 240 Russin Genève-Champel Gaillard 210 Lancy-Pont-Rouge Thônex Russin Dardagny Onex Challex -

Bibli-O-Bus Horaireshoraires Janvier Janvier À Décembre À Décembre 2018 2018 Informations Pratiques Bt Hermance
ap Céligny bibli-o-bus horaireshoraires janvier janvier à décembre à décembre 2018 2018 Informations pratiques bt Hermance S’inscrire : Collex-Bossy as am • L’inscription est gratuite pour tous les habitants du canton de Genève Anières sur présentation d’une pièce d’identité. Elle donne accès à l’ensemble du réseau des bibliothèques municipales bn at Corsier Collonge-Bellerive Emprunter, prolonger et réserver : co bs dp Gy Meinier Vésenaz • Tout emprunt nécessite la présentation de la carte d’emprunteur. bq Grand-Saconnex Elle est nominative et non transmissible. ck ar Jussy • Vous pouvez emprunter jusqu’à 20 documents et do Choulex 10 magazines sur l’ensemble du réseau pour une Vandoeuvres ct cn Presinge durée de 28 jours. dm dk Satigny Le Lignon Puplinge • Vos emprunts peuvent être prolongés à 3 reprises : bp - par e-mail : [email protected] Dardagny dl aq Chêne-Bourg - par téléphone : 022 418 92 70 (répondeur 24h/24) Russin al - sur le site des BM, via « mon espace » : cl Aire-la-Ville cp dn Cressy Onex Thônex - https://collectionsbmu.ville-ge.ch/Zones2/ La Plaine bl bm ao cr Bois-Gourmand Confignon • Si un document n’est pas présent dans le bus, vous Pinchat pouvez le réserver sur place, par e-mail ou téléphone cs br Grand-Salève (Veyrier) cm cq Plan-les-Ouates Laconnex Perly-Certoux an bk Compesières Bardonnex Croix-de-Rozonbo : A. Barman n illustratio Le Bibliobus vous propose un vaste choix d’ouvrages Retrouvez toutes les informations sur www.bm-geneve.ch pour adultes, enfants et adolescents : www.bm-geneve.ch @genevebm des romans, des documentaires, des livres en grands caractères, www.facebook.com/genevebm des bandes dessinées, des albums, et bien plus encore. -

Short Version
ACTIVITY REPORT Short 2018 version © TRIAL International/Sabrina Dangol © TRIAL International/Sabrina 15 YEARS OF ACTIVISM Dear friends, 20 years ago, in October 1998, Chilean dictator Augusto Pinochet was arrested in London for mass atrocities. Like many human rights activists, I remember this episode as a turning point for international justice. Suddenly, abstract legal concepts had teeth and could © Olivier Chamard bring a once-powerful ruler to account. Since then, the fight against impunity has evolved and expanded. It has been a rocky road with many drawbacks. But justice and accountability are undeniably gaining momentum: The awarding of the 2018 Nobel Peace Prize to Dr Mukwege and Nadia Murad for their fight against conflict-related sexual violence bears witness to unprecedented international attention. I founded TRIAL International shortly after Pinochet’s arrest. Just like the fight itself, our organization has grown and matured. Since its creation, it has supported over 2’300 victims and helped secure numerous decisions and judgments in their favor. This year, it finished second at the Financial Times Innovative Lawyers Awards. Our organization also kickstarted an ambitious cooperative project to fight impunity for conflict-related sexual violence worldwide. We have already come a long way, yet I am convinced that this is just the beginning! With your help we can go further and, just like we were inspired 20 years ago by Pinochet’s arrest, inspire others that justice is within reach. Philip Grant, Director Read our full activity report online at trialinternational.org/2018 2018 AT A GLANCE 724 31 325 new victims new cases legal practitioners supported trained 2’891’189 CHF 37 36 annual income staff field members missions TRIAL International is a non-governmental organization fighting impunity for international crimes and supporting victims in their quest for justice. -

Vitis Vinifera L. Ssp. Sativa) Based on Chloroplast DNA Polymorphisms
Molecular Ecology (2006) 15, 3707–3714 doi: 10.1111/j.1365-294X.2006.03049.x MultipleBlackwell Publishing Ltd origins of cultivated grapevine (Vitis vinifera L. ssp. sativa) based on chloroplast DNA polymorphisms R. ARROYO-GARCÍA,*† L. RUIZ-GARCÍA,* L. BOLLING,* R. OCETE,‡ M. A. LÓPEZ,‡ C. ARNOLD,§ A. ERGUL,¶ G. SÖYLEMEZO˝LU,** H. I. UZUN,†† F. CABELLO,‡‡ J. IBÁÑEZ,‡‡ M. K. ARADHYA,§§ A. ATANASSOV,¶¶ I. ATANASSOV,¶¶ S. BALINT,*** J. L. CENIS,††† L. COSTANTINI,‡‡‡ S. GORIS- LAVETS,§§§ M. S. GRANDO,‡‡‡ B. Y. KLEIN,¶¶¶ P. E. M C GOVERN,**** D. MERDINOGLU,†††† I. PEJIC,‡‡‡‡ F. PELSY,†††† N. PRIMIKIRIOS,§§§§ V. RISOVANNAYA,§§§ K. A. ROUBELAKIS-ANGELAKIS,§§§§ H. SNOUSSI,¶¶¶¶ P. SOTIRI,***** S. TAMHANKAR,††††† P. THIS,‡‡‡‡‡ L. TROSHIN,§§§§§ J. M. MALPICA,† F. LEFORT¶¶¶¶¶ and J. M. MARTINEZ-ZAPATER* *Departamento de Genética Molecular de Plantas, Centro Nacional de Biotecnología, CSIC, C/Darwin 3, 28049 Madrid, Spain, †Departamento de Biotecnología, INIA, Ctra. de A Coruña km 7, 28040 Madrid, Spain, ‡Departamento de Fisiología y Zoología, Universidad de Sevilla, C/Reina Mercedes 6, 41012 Sevilla, Spain, §National Center of Competence in Research 'Plant Survival', University of Neuchâtel, Emile Argand 11, Ch 2007, Neuchâtel, Switzerland, ¶Institute of Biotechnology, Ankara University, 065000 Besevler-Ankara, Turkey, **Department of Horticulture, Faculty of Agriculture, Ankara University, 06110 Diskapi-Ankara, Turkey, ††Department of Horticulture, Faculty of Agriculture, Akdeniz University, Antalya, Turkey, ‡‡Finca El Encin, IMIDRA, Alcalá de Henares, 28800 Madrid, Spain, §§National Clonal Germplasm Repository, USDA-ARS, University of California, Davis, CA 95616, USA, ¶¶AgroBioInstitute, D. Tzankov 8, 1164 Sofia, Bulgaria, ***Department of Viticulture, University of Agricultural Sciences and Veterinary Medicine ‘Ion Ionescu de la Brad’, Aleea Mihail Sadoveanu nr.