Proceedings of the National Workshop on Effects of Habitat Alteration on Salmonid Stocks
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Socioeconomic Monitoring of the Olympic National Forest and Three Local Communities
NORTHWEST FOREST PLAN THE FIRST 10 YEARS (1994–2003) Socioeconomic Monitoring of the Olympic National Forest and Three Local Communities Lita P. Buttolph, William Kay, Susan Charnley, Cassandra Moseley, and Ellen M. Donoghue General Technical Report United States Forest Pacific Northwest PNW-GTR-679 Department of Service Research Station July 2006 Agriculture The Forest Service of the U.S. Department of Agriculture is dedicated to the principle of multiple use management of the Nation’s forest resources for sustained yields of wood, water, forage, wildlife, and recreation. Through forestry research, cooperation with the States and private forest owners, and management of the National Forests and National Grasslands, it strives—as directed by Congress—to provide increasingly greater service to a growing Nation. The U.S. Department of Agriculture (USDA) prohibits discrimination in all its programs and activities on the basis of race, color, national origin, age, disability, and where applicable, sex, marital status, familial status, parental status, religion, sexual orientation, genetic information, political beliefs, reprisal, or because all or part of an individual’s income is derived from any public assistance program. (Not all prohibited bases apply to all pro- grams.) Persons with disabilities who require alternative means for communication of program information (Braille, large print, audiotape, etc.) should contact USDA’s TARGET Center at (202) 720-2600 (voice and TDD). To file a complaint of discrimination, write USDA, Director, Office of Civil Rights, 1400 Independence Avenue, SW, Washington, DC 20250-9410 or call (800) 795-3272 (voice) or (202) 720-6382 (TDD). USDA is an equal opportunity provider and employer. -

Pysht River Conservation Area
A Place Worth Getting There: From Port Angeles, drive about 3 miles west on Hwy 101. Turn right onto Hwy 112 Protecting and drive about 37 miles. The parking area is at milepost 24. North Olympic Land Trust is your community nonprofit dedicated to the conservation of open spaces, local food, local resources, healthy watersheds and recreational opportunities. Our long-term Pysht River goal and mission is to conserve lands that sustain the communities of Clallam County. Conservation Area Since 1990, North Olympic Land Trust has permanently conserved: More than 520 acres of local farmland 12 miles of stream and river habitat More than 1,800 602 EAST FRONT ST. P.O. BOX 2945 (MAILING) acres of forestland PORT ANGELES, WASH I NGTON 98362 (360) 417-1815 FOR THE LATEST NEWS | TO EXPLORE THE LAND | TO DONATE: [email protected] northolympiclandtrust.org northolympiclandtrust.org Pysht River Habitat Restoration The Pysht River Conservation Area, located 8.7 miles from the mouth of the Pysht River, protects 74 acres of land, including 2/3 mile of the Pysht River, 1,500 feet of Green Creek and four wetlands. The Pysht River is used by coho salmon, cutthroat trout, and steelhead. The Area also is vital for the recovering productivity of chinook and chum salmon. The Makah Tribe led initial restoration efforts within the Area by removing the dilapidated structures and non-native invasive vegetation, and with the help of the Lower Elwha Klallam Tribe, re-planted over 7,000 native trees. Further plantings were completed in partnership with the Clallam Conservation District, USDA Farm Service Agency and Merrill & Ring. -

Watershed Plan
CHAPTER 1. INTRODUCTION AND BACKGROUND This watershed plan for the Hoko-Lyre Watershed provides a comprehensive review and evaluation of vital water resources in Water Resource Inventory Area (WRIA) 19 and lays the groundwork for future management and stewardship of these resources. Located on the Olympic Peninsula (see Figure 1-1), WRIA 19 is a beautiful and remote area with few human inhabitants, though it carries a legacy of large- scale logging throughout the region. Based on the review of water resources, this plan outlines steps for ensuring the optimum ongoing use of the watershed’s surface waters and groundwater in a way that balances water needs for human use and environmental protection. An overview of the important characteristics of WRIA 19 is provided below. Appendix A provides more detailed descriptions of WRIA 19 features that are important for consideration in a watershed plan. Figure 1-1. WRIA 19 and Subbasins 1.1 WHY WAS THIS PLAN DEVELOPED? In 1998, the Washington State Legislature created the Watershed Management Act (Revised Code of Washington (RCW) 90.82) to support local communities in addressing water resource management issues. The act established a voluntary watershed management planning process for the major river basins in the state. The goal of the planning process is to support economic growth while promoting water availability and quality. The Act encourages local governments and interested groups and citizens to assess basin water resources and develop strategies for managing them. The Washington State Department of Ecology (Ecology) defined boundaries that divide the state into WRIAs, which correspond to the watersheds of major rivers, and established funding for groups in each WRIA that choose to undertake the planning process (funding is broken down by phases of the planning 1-1 WRIA 19 Watershed Plan… effort, as described in Appendix B). -

Proposed Ranked List of Projects for 2015-17 Capital Budget Funding
Work on the Puyallup River Proposed Ranked List of Projects for 2015-17 Capital Budget Funding Ecology Publication #: 14-06-033 October 2014 / revised February 2019 Proposed Ranked Projects for 2015-17 Capital Budget Funding I,Whitma _,.,J n D C PA R I M E N I O F Projects above $50 million total ECOLOGY State of Wa>h ington DProjects below $50 million total Sources: NASA, USGS, ESRI,NAIP,Washington State Orthoportal, other suppliers Ecology Publication #: 14-06-033 October 2014 / revised February 2019 Ecology FY 2015-17 Proposed Floodplain by Design Project List Rank Project Description Grant Local Project Legis Request Match Total Dist. Yakima FP Management Program: Rambler's Park Phase IV and Trout 1 Meadows Phase II (Yakima County) $2,358,000 $592,000 $2,950,000 15 Puyallup Watershed Floodplain 2 Reconnections - Tier 1 (Pierce County) $10,240,000 $2,544,250 $12,784,250 31 Lower Dungeness River Floodplain 3 Restoration (Clallam County) $9,501,600 $2,375,400 $11,877,000 24 Boeing Levee/Russell Road Improvements & Floodplain Restoration 4 (King County Flood & Control District) $4,900,000 $24,400,000 $29,300,000 33 South Fork Nooksack - Flood, Fish and Farm Conservation (Whatcom Land 5 Trust) $3,216,958 $811,090 $4,028,048 42 Middle Green River/Porter Gateway Protection and Restoration (King 6 County) $3,648,926 $1,737,373 $5,386,299 31 Cedar River Corridor Plan 7 Implementation (King County) $5,000,000 $3,000,000 $8,000,000 5 Sustainable Management of the Upper Quinault River Floodplain (Quinault 8 Indian Nation) $560,000 $140,000 $700,000 -

Tribal Implementation Final Report
Puget Sound National Estuary Program: Tribal Implementation Award PA-00J32201: FY10-13 Final report March 15, 2018 Contact: Dani Madrone Puget Sound Recovery Projects Coordinator, NWIFC [email protected] 360-528-4318 This project has been funded wholly or in part by the United States Environmental Protection Agency under assistance agreement PA-00J32201 to the Northwest Indian Fisheries Commission. The contents of this document do not necessarily reflect the views and policies of the Environmental Protection Agency, nor does mention of trade names or commercial products constitute endorsement or recommendation for use. EPA Award PA-00J32201: FY10-13 Final report Introduction On behalf of the federally recognized tribes of Puget Sound, the Northwest Indian Fisheries Commission (NWIFC) developed a program to administer the Environmental Protection Agency (EPA) National Estuary Program award dedicated to tribal restoration and protection projects in the Puget Sound watershed. These funds were awarded under a cooperative agreement (PA-00J32201), under which NWIFC served as the Lead Organization (LO) for the tribal distribution of these funds. This report details the outcomes, successes, and reflections of this first cooperative agreement, which included four awards that spanned federal fiscal years 2010- 13 and closed December 31, 2017, and offers a forward looking approach on the continuation of tribal implementation projects for Puget Sound under this program. Overview of Approach As the Lead Organization (LO) for the tribal distribution of the National Estuary Program award for Puget Sound, NWIFC has a unique, non-competitive approach to the allocation of this award. The cooperative agreement between NWIFC and EPA Region 10 recognizes the federal government’s trust responsibility to each of the federally recognized Indian tribes within the region. -

NOPLE Work Plan 2016
22001166 WWoorrkk PPllaann North Olympic Peninsula Lead Entity for Salmon Clallam County Courthouse 223 E. Fourth Street, # 5 Port Angeles, WA 98362 360/417-2326 1 NOPLE Work Plan 2016 Table of Contents Introduction .................................................................................................................................................. 3 Membership ................................................................................................................................................. 5 List of Ranked 2016 Work Plan Narratives .................................................................................................. 7 2016 Narrative Ranking Workbook ........................................................................................................... 18 Capital Scoring Criteria ............................................................................................................................ 30 Non-Capital Scoring Criteria .................................................................................................................... 33 2016 Review of Narrative Ranking ............................................................................................................ 36 Project Narratives....................................................................................................................................... 38 2 NOPLE Work Plan 2016 North Olympic Peninsula Lead Entity for Salmon Cl allam County Courthouse February, 2016 223 E. Fourth Street, # 5 Port Angeles, -

Lower Elwha S'klallam Tribe
ELWHA FISHERIES OFFICE 760 Stratton Rd (360) 457-4012 Port Angeles, WA 98363 FAX: (360) 452-4848 November 29, 2018 LOWER ELWHA KLALLAM TRIBE Steelhead Regulation F18-058 The following regulations are promulgated by the Lower Elwha Klallam Tribe and cover commercial and subsistence fishing for steelhead salmon in the on and off-reservation areas as outlined below: SECTION I. GENERAL PROVISIONS TOPIC 1. MANAGEMENT AREAS These regulations shall apply to the following on and off- reservation areas by Lower Elwha tribal fishers: A. Marine Areas Areas 4B, 5, 6, 6A, 6B, 6C, 6D (outside of the Jamestown S’Klallam exclusive harvest zone), 7, 9, 12, 12A & 12B. B. Freshwater Areas Elwha R., Hoko R., Clallam R., Pysht R., Deep Crk, E. & W. Twin R., Salt Crk., Lyre R., Morse Crk., Dungeness R., Dosewallips., Duckabush R., Big Beef Creek, Big Quilcene R., and Hamma Hamma R. TOPIC 2. LAWFUL GEAR Whenever any fishery openings are promulgated, the following shall be considered lawful gear. A. Marine Areas a) Drift gillnets (min. mesh 5 inches); length as specified in the Lower Elwha Klallam Fishing Ordinance 3rd Edition. 1 b) Set gillnets (min. mesh 5 inches; length in Area 6D 100 fms.; elsewhere, 150 fms.). c) Hook and line gear as specified in the Lower Elwha Klallam Fishing Ordinance 3rd Edition. B. Rivers a) Set gillnets (min. mesh 5 inches; max. length 20 fms). The Elwha River (min. mesh 5 inches; max. length 25 fms). b) Hook and line gear as specified in the Lower Elwha Klallam Fishing Ordinance 3rd Edition. TOPIC 3. -

Marine Reaches
Clallam County SMP Update - Inventory and Characterization Report 4. MARINE REACH INVENTORY This chapter describes the marine shoreline reaches that are within the jurisdiction of the County’s SMP (in WRIAs 18, 19, and a portion of 17, excluding incorporated areas and the Makah Reservation) (see Figure 3-7 for the reach locations). Reaches are described in terms of their physical attributes, ecological condition, and human environment / land use characteristics. Maps are provided in Appendix A. Based upon available County-wide data sources, key physical, ecological, and land use characteristics for each reach are detailed on “reach sheets,” located at the end of this section. A description of the available data sources, including data limitations, is presented in the “reach sheet explainer” following this chapter. The reach descriptions below contain a summary of the data presented within the reach sheets and additional pertinent information, including potential future land use impacts to shoreline processes and management issues and opportunities. 4.1 Reach 1: Diamond Point (Maps 1a to 6a in Appendix A) The “Diamond Point” reach contains 12.5 miles of marine shoreline, which extends along Miller Peninsula from the Clallam/Jefferson county line to the northwest corner of Sequim Bay (approximately one mile south of Travis Spit). The reach contains shoreline along Discovery Bay, the Strait of Juan de Fuca, and Sequim Bay. The reach also contains the mouth of Eagle Creek (Eagle Creek is not a shoreline of the state, except where it enters the Strait of Juan de Fuca). Jefferson County has designated the majority of the adjacent Discovery Bay shorelands as either Natural or Conservancy. -
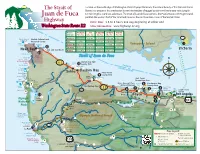
Highway Drive Time: 1.5 to 2 Hours One Way Beginning at Either End Washington State Route 112 More Information
Located on the north edge of Washington State’s Olympic Peninsula, the natural beauty of this National Scenic The Strait of Byway is as unique as it is spectacular. Its remote stretches of rugged coastline will make your ride along its Juan de Fuca 61-mile length a stand-out adventure. The Strait of Juan de Fuca connects the Pacic Ocean with Puget Sound, parallels the western half of the Strait and traverses the northwestern corner of the United States. Highway Drive Time: 1.5 to 2 hours one way beginning at either end Washington State Route 112 More information: www.highway112.org Distances Port Angeles Joyce Cl. Bay/Sekiu Lake Ozette Neah Bay & Mileages Miles / Time Miles / Time Miles / Time Miles / Time Miles / Time 17 Tatoosh Island & Makah Cultural and Port Angeles 16/24 min. 50/1 hr.16 min. 75/2hrs.7 min. 68/1 hr.47 min. Lighthouse Research Center Joyce 16/24 min. 34/53 min. 59/1 hr.45 min. 54/1 hr.20 min. Cape Flattery Neah Bay Cl. Bay/Sekiu 50/1 hr.16 min. 34/53 min. 25/50 min. 20/32 min. Vancouver Island 12 Lake Ozette 75/2hrs.7 min. 59/1 hr.45 min. 25/50 min. 38/1 hr.11 min. Neah Bay Sail and Seal Rocks Victoria Neah Bay 68/1 hr.47 min. 54/1 hr.20 min. 75/2hrs.7 min. 38/1 hr.11 min. Au to and P Hobuck Beach 13 Makah Strait of Juan de Fuca Makah Bay Reservation assenger-Only F Flattery Rocks Sooes Ri National Wildlife Clallam Bay Spit Refuge Sekiu 112 Point ve 10 County Park Shi Shi Beach r Clallam Bay Point of the Arches erries er Clallam Bay Ozette Indian Sekiu Pillar Point o Riv Village Hok 9 County Park Archaeological Pysht -

Sub-Region: Western Strait (See Map with Habitat Complexes and Drift)
Appendix B – 1: Western Strait Sub-Region Table of Contents Sub-Region Summary ……………………………………………………………. 2 Geographic Location …………………………………………………….. 2 Geology and Shoreline Sediment Drift …………………………………. 2 Information Sources …………………………………………………….. 3 Description of Sub-regional Habitat Complexes ………………………. 4 Habitat Changes and Impairment of Ecological Processes …………… 7 Relative Condition of Habitat Complexes ……………………………… 7 Management Recommendations …………………………………………8 Habitat Complex Narratives …………………………………………………….10 Neah Bay ………………………………………………………………….10 Sail River ………………………………………………………………….18 Snow Creek ……………………………………………………………… 20 Bullman Creek ……………………………………………………………22 Rasmussen, Jansen, and Olsen creeks …………………………………. 24 Sekiu River ………………………………………………………………. 24 Hoko River ………………………………………………………………. 28 Clallam River ……………………………………………………………. 36 References…………………………………………………………………………45 1 Western Strait Sub-Region Sub-Region Summary Geographic Location The Western Strait sub-region extends from Koitlah Point that marks the west end of Neah Bay (west of the breakwater) to an area of “no appreciable drift” along rocky headlands located between Clallam Bay and the Pysht River (Figure 1). Shorelines in the Western and Central Strait sub-regions alternate between relatively protected pockets (largest ones being Neah Bay, Clallam Bay, Pysht, and Crescent Bay) that tend to accumulate sediment material sometimes derived from fluvial sources, and the more exposed and often very steep rocky shorelines. Geology and Shoreline Sediment Drift Geologically, -

Nearshore Fish and Macroinvertebrate Assemblages
Administration United States National Oceanic and Atmospheric Laboratories Department of Environmental Research Joe Commerce Seattle WA 98115 L lie EPA 600 7 80 027 United States Office of Environmental Environmental Protection Engmeering and Technology January 1980 DC 20460 PA Agency Washington Researdl and Development Nearshore Fish and Macroinvertebrate Assemblages Along the Strait of Juan de Fuca Including Food Habits of the Common Nearshore Fish Interagency Energy Environment R D Program Report 4lII i J I I 1 I i I 11 n I RESEARCH REPORTING SERIES I Research reports of the Office of Research and Development U S Environmental Protection a f Agency have been grouped into nine series These nine broad cate gories were established to facilitate further development and application of en vironmental technology Elimination of traditional grouping was consciously planned to foster technology transfer and a maximum interface in related fields a I The nine series are I I 1 Environmental Health Effects Research I 2 Environmental Protection Technology o I 3 Ecological Research 4 Environmental Monitoring 5 Socioeconomic Environmental Studies o 6 SCientific and Technical Assessment Reports STAR 7 Interagency Energy Environment Research and Development 8 Special Reports o 9 Mi scellaneous Repons This report has been assigned to the INTERAGENCY ENERGY ENVIRONMENT RESEARCH AND DEVELOPMENT series Reports in this series result from the o effort funded under the 17 agency Federal Energy Environment Research and Development Program These studies relate -

Marine Resource Damage Assessment Report for the ARCO Anchorage Oil Spill December 21 1985 Into Port Angeles Harbor and the Strait of Juan De Fuca
i L 1 bc itvf ll Lasered CED 5 FFe 0 t 1 c r 1 MARlNE R ESOURCE 1 r I v r 1 i DAMAGE NT T I i ASSESSME REPOR t f f l 1 I 4 1 FOR THE ARCO A CHORAGE Oil SPilt r r 1 I j c 1 I fi r j w c 1 h 0 J 4 l 11 c w i f 1 I 1 1 t I J H c lt 1 1 j i t 4 0 J t It j t ii i F l 1 t 1 v l r e cT Stateof t 4 Wlshington t l 1 1 l 0 f f j Ecology JANUARY 1987 87 4 The Washington State Department of Ecology Marine Resource Damage Assessment Report for The ARCO Anchorage Oil Spill December 21 1985 into Port Angeles Harbor and the Strait of Juan de Fuca by Lewey J Kittle Jr Project Leader Richard Burge Randy Butler Walter Cook Kurt Fresh Michea1 Kyte Richard Lowell Richard Pease Dan Penttila Al Scholz Steven Speich Robert Stee1quist Jim Walton January 1987 87 4 ACKNOWLEDGMENTS AJ the ARCO Anchorage MROA project leader I would like to thank all the m mbers of this team for their time and effort For the majority this pfoject was not a part of their regular duties and took a special effort e T team worked to accomplish this task and from my perspective it was The is a list MRDA g I nuinely appreciated following of the Team members I Dlck Burge WDF Shellfish Biologist A1 Scholz WDF Shellfish Biologist Earl Finn WDF Fisheries Biologist MRDA agency liaison Dan Penttila WDF Fisheries Biologist Dick Pease WDF Shellfish Biologist Lynn Goodwin WDF Shellfish Biologist Walt Cook WDF Shellfish Biologist Randy Butler WDF Shellfish Biologist Jim Walton Peninsula College 8Gb Steelquist Peninsula College Jim Beam WDF Fisheries Biologist Kurt Fresh WDF Fisheries Biologist B