Fertigation Chemicals Presented At
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Mechanosynthesis of Magnesium and Calcium Salt?Urea Ionic Cocrystal
Letter pubs.acs.org/journal/ascecg Mechanosynthesis of Magnesium and Calcium Salt−Urea Ionic Cocrystal Fertilizer Materials for Improved Nitrogen Management Kenneth Honer, Eren Kalfaoglu, Carlos Pico, Jane McCann, and Jonas Baltrusaitis* Department of Chemical and Biomolecular Engineering, Lehigh University, B336 Iacocca Hall, 111 Research Drive, Bethlehem, Pennsylvania 18015, United States *S Supporting Information ABSTRACT: Only 47% of the total fertilizer nitrogen applied to the environment is taken up by the plants whereas approximately 40% of the total fertilizer nitrogen lost to the environment reverts back into unreactive atmospheric dinitrogen that greatly affects the global nitrogen cycle including increased energy consumption for NH3 synthesis, as well as accumulation of nitrates in drinking water. In this letter, we provide a mechanochemical method of inorganic magnesium and calcium salt−urea ionic cocrystal synthesis to obtain enhanced stability nitrogen fertilizers. The solvent-free mechanochemical synthesis presented can result in a greater manufacturing process sustainability by reducing or eliminating the need for solution handling and evaporation. NH3 emission testing suggests that urea ionic cocrystals are capable of decreasing NH3 emissions to the environment when compared to pure urea, thus providing implications for a sustainable global solution to the management of the nitrogen cycle. KEYWORDS: Fertilizers, Nitrogen, urea, Mechanochemistry, Cocrystal, pXRD, NH3 Emissions, Stability ■ INTRODUCTION ammonia as opposed to up to 61.1% of soil treated with urea 7,8 fi only, which suggests that major improvements to the global Atmospheric dinitrogen, N2, xation to synthesize ammonia, 9,10 ’ 1 nitrogen cycle are achievable. Additionally, urea molecular NH3, consumes more than 1% of the world s primary energy. -

Naturally Chelated Foliar Blends
Fertigation for citrus trees By Mongi Zekri, Brian Boman and Tom Obreza icroirrigation is an important with overhead sprinkler irrigation. component of citrus produc- Research has also shown the impor- Mtion systems in Florida. For tant advantage of microsprinklers for citrus trees, microirrigation (Figure 1) freeze protection of citrus. is more desirable than other irrigation Microirrigation combined with methods for several reasons: water properly managed, water savings with fertigation (Figure 2) — applying conservation, fertilizer manage- microirrigation systems can amount to small amounts of soluble fertilizer ment efficiency and freeze protec- as much as 80 percent compared with through irrigation systems directly tion. Research has shown that when subirrigation and 50 percent compared to the root zone — provides precise Figure 1. Microsprinkler irrigation of citrus trees. Figure 2. Fertigation system for citrus trees. Naturally Chelated ORDER Foliar Blends ONLINE Clear natural chelates for higher analysis, better results and affordable prices. www.moreoranges.com (866) 375-2487 14 CITRUS INDUSTRY • March 2014 Figure 3. Fertigation system including backflow prevention devices. timing and application of water and ces are in place and working properly. solution should be mixed with irriga- fertilizer nutrients in citrus production. The time required for water to tion water in a jar (at the same dilu- Fertilizer can be prescription-applied travel from the injection point to the tion rate that is used in the irrigation in small doses and at particular times farthest emitter is generally 20 to 30 system) to determine if any precipitate when those nutrients are needed. This minutes for most microirrigation sys- or milkiness occurs within one to two capability helps growers increase tems. -

Growwithpeters.Com © 2020 ICL Fertilizers, Worldwide Rights Reserved
PRODUCT ANALYSIS AND RATES IDEAL FOR WATER TYPES 1 to 2 1 2 3 4 0 60 150 200 240+ All-Purpose Low High 15-2-20 PPM CALCIUM CARBONATE Formulation Alkalinity Alkalinity PANSY, SALVIA & VINCA SKU# E99130, G99130 Formulated for compact growth and bright blossoms on WEIGHT (IN OUNCES) OF PRODUCT NEEDED TO MIX pansy, salvia, vinca, etc . with high Nitrate, low Phosphate ONE GALLON OF CONCENTRATE and extra Boron . Target Fertilizer Common Injector Ratios EC (mmhos/cm) • A (All-Purpose) formulation for constant, balanced Concentration of Target Feed nutrition (N/ppm) After 1:15 1:100 1:128 1:200 1:300 Rate After • Most effective with Water Types 1 and 2 Dilution Dilution • Contains Calcium, Magnesium and other minor 25 0 .3 2 .3 2 .9 4 .5 6 .8 0 .21 elements 50 0 .7 4 .5 5 .8 9 .0 13 .5 0 .42 GUARANTEED ANALYSIS 15-2-20 75 1 .0 6 .8 8 .6 13 .5 20 .3 0 .62 Total Nitrogen (N) . .. 15% 100 1 .4 9 .0 11 .5 18 .0 27 .0 0 .83 1 .4% Ammoniacal Nitrogen 12 .8% Nitrate Nitrogen 125 1 .7 11 .3 14 .4 22 .5 33 .8 1 .04 0 .8% Urea Nitrogen 150 2 .0 13 .5 17 .3 27 .0 40 .5 1 .25 Available Phosphate (P2O5) . 2% 175 2 .4 15 .8 20 .2 31 .5 47 .3 1 .45 Soluble Potash (K2O) . 20% Calcium (Ca) . 3 .75% 200 2 .7 18 .0 23 .0 36 .0 54 .0 1 .66 Magnesium (Mg) . -

Ammonium Thiosulfate, Solution ((NH4)2S2O3) Safety Data Sheet According to Federal Register / Vol
Ammonium Thiosulfate, Solution ((NH4)2S2O3) Safety Data Sheet According To Federal Register / Vol. 77, No. 58 / Monday, March 26, 2012 / Rules And Regulations And According To The Hazardous Products Regulation (February 11, 2015). Revision Date: 04/03/2019 Date of Issue: 01/22/2014 Supersedes Date: 03/08/2017 Version: 2.1 SECTION 1: IDENTIFICATION 1.1. Product Identifier Product Form: Mixture Product Name: Ammonium Thiosulfate, Solution ((NH4)2S2O3) Synonyms: Ammonium Hyposulfite, Solution 1.2. Intended Use of the Product No use is specified. 1.3. Name, Address, and Telephone of the Responsible Party Company Manufacturer Poole Chemical Co., Inc. Poole Chemical Co., Inc. P.O. Box 10 P.O. Box 10 100 N. 1st Street 100 N. 1st Street Texline, TX 79087 - United States Texline, TX 79087 - United States T 806-362-4261 T 806-362-4261 1.4. Emergency Telephone Number Emergency Number : 1-800-424-9300 (CHEMTREC) SECTION 2: HAZARDS IDENTIFICATION 2.1. Classification of the Substance or Mixture GHS-US/CA Classification Not classified 2.2. Label Elements GHS-US/CA Labeling No labeling applicable 2.3. Other Hazards Exposure may aggravate pre-existing eye, skin, or respiratory conditions. 2.4. Unknown Acute Toxicity (GHS-US/CA) No data available SECTION 3: COMPOSITION/INFORMATION ON INGREDIENTS 3.1. Substance Not applicable 3.2. Mixture Name Synonyms Product Identifier % * GHS Ingredient Classification Ammonium thiosulfate Ammonium thiosulphate / Thiosulfuric acid, (CAS-No.) 7783-18-8 60 Not classified diammonium salt / Thiosulfuric acid (H2S2O3), diammonium salt / Thiosulfuric acid (H2S2O3), ammonium salt (1:2) / Diammonium thiosulfate Water AQUA / Aqua (CAS-No.) 7732-18-5 40 Not classified *Percentages are listed in weight by weight percentage (w/w%) for liquid and solid ingredients. -

Improved Solubility Compound Fertilizer
Europaisches Patentamt (19) European Patent Office Office europeenpeen des brevets EP 0 569 513 B1 (12) EUROPEAN PATENT SPECIFICATION (45) Date of publication and mention (51) intci.6: C05B 17/02, C05C 5/04, of the grant of the patent: C05D 9/02, C05G 1/00 12.11.1997 Bulletin 1997/46 (86) International application number: (21) Application number: 92905776.8 PCT/US92/00850 Date of 31.01.1992 (22) filing: (87) International publication number: WO 92/13813 (20.08.1992 Gazette 1992/22) (54) IMPROVED SOLUBILITY COMPOUND FERTILIZER COMPOSITIONS Losliche Dungemittelzusammenstellungen COMPOSITIONS POUR ENGRAIS SOUS FORME DE COMPOSES SOLIDES A SOLUBILITE ACCRUE (84) Designated Contracting States: (74) Representative: De Hoop, Eric et al AT BE CH DE DK ES FR GB GR IT LI LU MC NL SE Octrooibureau Vriesendorp & Gaade P.O. Box 266 (30) Priority: 31.01.1991 US 648644 2501 AW Den Haag (NL) (43) Date of publication of application: (56) References cited: 18.11.1993 Bulletin 1993/46 GB-A- 2 072 644 (60) Divisional application: 97200001.2 • Chemical Abstracts, volume 93, no. 11,15 September 1980, (Columbus, Ohio, US) see page (73) Proprietor: OMS INVESTMENTS, Inc. 622, abstract 113193d, & PI 7908335 Wilmington, Delaware 19801 (US) (ULTRAFERTIL S.A.) 04-03-1980 (72) Inventors: Remarks: • VETANOVETZ, Richard, P. •Divisional application 97200001 .2 filed on Emmaus, PA 18049 (US) 03/01/97. • PETERS, Robert •The file contains technical information submitted Allentown, PA 18104 (US) after the application was filed and not included in this specification DO CO lo O) CO LO Note: Within nine months from the publication of the mention of the grant of the European patent, any person may give notice the Patent Office of the Notice of shall be filed in o to European opposition to European patent granted. -

Ammonium Thiosulfate 12-0-0, 26% Sulfur
Secondary Nutrients Ammonium Thiosulfate 12-0-0, 26% Sulfur Guaranteed Analysis Directions for Use: Greens, Tees and Fine Turf: Apply 3.0 - 12.0 oz. of Ammonium Thiosulfate with 1.5 -2 gallons of Total Nitrogen (N) ................................... 12.00% water per 1,000 sq. ft. (1.0 - 4.1 gallons of Ammonium Thiosulfate with 66 - 88 gallons of water per 12% Ammoniacal Nitrogen Sulfur (S) ................................................. 26.00% Acre) every 14 days throughout the growing season. Irrigate after application. This application shall provide 0.03 - 0.12 lb. of actual Nitrogen per 1,000 sq. ft. Derived from Ammonium Thiosulfate Fairways, Roughs, Sports Turf and Lawns: Apply 3.1 - 4.1 gallons of Ammonium Thiosulfate Ammonium Thiosulfate is an excellent source with 44 - 88 gallons of water per Acre (9.0 - 12.0 oz. of Ammonium Thiosulfate with 1 - 2 gallons of of ammoniacal nitrogen that is quickly absorbed water per 1,000 sq. ft.) every 14 days throughout the growing season. This application shall provide by the plant. This results in greener turf, even at 0.09 - 0.12 lb. of actual nitrogen per 1,000 sq. ft. low soil temperatures. Use when a liquid type of ammoniacal nitrogen source and sulfur are Fertigation: Apply 1.0 - 5.0 gallons per Acre (3 - 15 oz. per 1,000 sq. ft.) of Ammonium Thiosulfate required. with the irrigation water every 7 to 14 throughout the growing season. Ammonium Thiosulfate is a neutral to slightly Application Precautions: basic (7 - 8 pH), clear liquid solution, containing 12% nitrogen and 26% sulfur. Ammonium Do not apply Ammonium Thiosulfate directly on or below germinating seeds such as in a “pop Thiosulfate is compatible with most liquid up” fertilizer. -

Orca Corrosion Chart
Unsaturated Polyester Vinylster (Epoxy Acrylate Resins) CHEMICAL Conc Resins NO ISO BIS Novolac Bromine ENVIRONMENT % 511/512 301 585 570 545/555 A 1 Acetaldehyde 20 NR 40 40 40 2 Acetic Acid 10 80 100 100 100 3 Acetic Acid 15 60 100 100 100 4 Acetic Acid 25 60 100 100 100 5 Acetic Acid 50 - 80 80 80 6 Acetic Acid 75 NR 65 65 65 7 Acetic Acid, Glacial 100 NR NR 40 NR 8 Acetic Anhydride 100 NR NR 40 NR 9 Acetone 10 NR NR 80 80 10 Acetone 100 NR NR NR NR 11 Acetonitrile 20 - 40 40 40 12 Acetyl Acetone 20 - 40 50 40 13 Acrolein (Acrylaldehyde) 20 - 40 40 40 14 Acrylamide 50 NR 40 40 40 15 Acrylic Acid 25 NR 40 40 40 16 Acrylic Latex All - 80 80 80 17 Acrylonitrile Latex Dispersion 2 NR 25 25 25 Activated Carbon Beds, Water 18 - 80 100 80 Treatment Adipic Acid(1.5g solution in 19 23 - 80 80 80 water at 25℃, sol in hot water) 20 ALAMINE amines - 65 80 65 21 Alkyl(C8-10) Dimethyl Amine 100 - 80 100 80 22 Alkyl(C8-10) Chloride All - 80 100 95 23 Alkyl Benzene Sulfonic Acid 90 NR 50 50 50 Alkyl Tolyl Trimethyl 24 - - 40 50 40 Ammonium Chloride 25 Allyl Alcohol 100 NR NR 25 NR 26 Allyl Chloride All NR 25 25 25 27 Alpha Methylstyrene 100 NR 25 50 25 28 Alpha Oleum Sulfates 100 NR 50 50 50 29 Alum Sat'd 80 100 120 100 30 Aluminum Chloride Sat'd 80 100 120 100 31 Aluminum Chlorohydrate All - 100 100 100 32 Aluminum Chlorohydroxide 50 - 100 100 100 33 Aluminum Fluoride All - 25 25 25 34 Aluminum Hydroxide 100 80 80 95 80 35 Aluminum Nitrate All 80 100 100 100 36 Aluminum Potassium Sulfate Sat'd 80 100 120 100 37 Aluminum Sulfate Sat'd 80 100 120 100 -

Safety Data Sheet
SAFETY DATA SHEET SECTION 1) CHEMICAL PRODUCT AND SUPPLIER'S IDENTIFICATION Product ID: Ammonium Thiosulfate Product Name: Ammonium Thiosulfate Revision Date: Jul 13, 2015 Date Printed: Sep 29, 2015 Version: 2.0 Supersedes Date: Jun 05, 2015 Manufacturer's Name: Martin Operating Partnership, L.P. Address: P.O. Box 191, Kilgore, TX, US, 75663 Emergency Phone: CHEMTREC (800) 424-9300 Information Phone: 800-231-4595 Fax: Product/Recommended Uses: Industrial uses SECTION 2) HAZARDS IDENTIFICATION Classification: Acute toxicity, Oral - Category 4 Pictograms: Signal Word: Warning Hazardous Statements - Health: Harmful if swallowed Precautionary Statements - General: If medical advice is needed, have product container or label at hand. Keep out of reach of children. Read label before use. Precautionary Statements - Prevention: Wash with soap and water thoroughly after handling. Do not eat, drink or smoke when using this product. Precautionary Statements - Response: IF SWALLOWED: Call a POISON CENTER/doctor if you feel unwell. Rinse mouth. Precautionary Statements - Storage: No precautionary statement available. Precautionary Statements - Disposal: Dispose of contents/container to disposal recycling center. Under RCRA it is the responsibility of the user of the product to determine at the time of disposal whether the product meets RCRA criteria for hazardous waste. Waste management should be in full compliance with federal, state and local laws. Ammonium Thiosulfate www.martinresources.com Page 1 of 7 SECTION 3) COMPOSITION / INFORMATION ON INGREDIENTS CAS Chemical Name % By Weight 0007783-18-8 AMMONIUM THIOSULFATE 48% - 66% 0007732-18-5 WATER 34% - 46% 0007783-20-2 AMMONIUM SULFATE 0.9% - 2% 0010196-04-0 AMMONIUM SULFITE 0.1% - 2% SECTION 4) FIRST-AID MEASURES Inhalation: Remove source of exposure or move person to fresh air and keep comfortable for breathing. -

Effect of Surface Application of Ammonium Thiosulfate on Field
Science of the Total Environment 580 (2017) 316–323 Contents lists available at ScienceDirect Science of the Total Environment journal homepage: www.elsevier.com/locate/scitotenv Effect of surface application of ammonium thiosulfate on field-scale emissions of 1,3-dichloropropene S.R. Yates a,⁎, D.J. Ashworth a,b,Q.Zhanga a USDA-ARS, U.S. Salinity Laboratory, 450 W. Big Springs Rd., Riverside, CA 92507, United States b University of California, Department of Environmental Sciences, Riverside, CA 92521, United States HIGHLIGHTS GRAPHICAL ABSTRACT • Five methods and two independent da- ta sets were used to calculate emissions • Total 1,3-dichloropropene mass loss was 18.4 ± 6.7% and ranged 12–26% • Spraying ammonium thiosulfate fertil- izer on soil reduced 1,3-D emissions • Results compared to four related large- scale field experiments article info abstract Article history: Soil fumigation is important for food production but has the potential to discharge toxic chemicals into the environ- Received 29 September 2016 ment, which may adversely affect human and ecosystem health. A field experiment was conducted to evaluate the Received in revised form 17 November 2016 effect of applying ammonium thiosulfate fertilizer to the soil surface prior to fumigating with 1,3-dichloropropene Accepted 17 November 2016 (1,3-D). The ammonium thiosulfate solution was applied as a spray with minimal water to minimize the effect on Available online 21 December 2016 emissions from saturating (e.g. sealing) the soil pores with water. Two independent data sets were collected for de- Editor: Jay Gan termining the emission rate. One data set was used with three micrometeorological approaches: aerodynamic, inte- grated horizontal flux and theoretical profile shape; the other dataset with two indirect, back calculation methods Keywords: that used the CALPUFF and ISCST3 dispersion models. -

Ammonium Thiosulfate MSDS
Material Safety Data Sheet NFPA HMIS Personal Protective Equipment Health Hazard 0 1 1 0 Fire Hazard 0 Reactivity 0 See Section 15. Section 1. Chemical Product and Company Identification Page Number: 1 Common Name/ Ammonium Thiosulfate Catalog A1273 Trade Name Number(s). CAS# 7783-18-8 Manufacturer SPECTRUM LABORATORY PRODUCTS INC. RTECS XN6465000 14422 S. SAN PEDRO STREET TSCA TSCA 8(b) inventory: GARDENA, CA 90248 Ammonium Thiosulfate Commercial Name(s) Thio-sul CI# Not available. Synonym Diammonium Thiosulfate; Ammonium Hyposulfite IN CASE OF EMERGENCY Chemical Name Thiosulfuric acid, diammonium salt CHEMTREC (24hr) 800-424-9300 Chemical Family Not available. CALL (310) 516-8000 Chemical Formula H8-N2.O3-S2 Supplier SPECTRUM LABORATORY PRODUCTS INC. 14422 S. SAN PEDRO STREET GARDENA, CA 90248 Section 2.Composition and Information on Ingredients Exposure Limits Name CAS # TWA (mg/m3) STEL (mg/m3) CEIL (mg/m3) % by Weight 1) Ammonium Thiosulfate{2} 7783-18-8 100 Toxicological Data Not applicable. on Ingredients Section 3. Hazards Identification Potential Acute Health Effects Slightly hazardous in case of skin contact (irritant), of eye contact (irritant), of ingestion, of inhalation. Potential Chronic Health CARCINOGENIC EFFECTS: Not available. Effects MUTAGENIC EFFECTS: Not available. TERATOGENIC EFFECTS: Not available. DEVELOPMENTAL TOXICITY: Not available. Repeated or prolonged exposure is not known to aggravate medical condition. Continued on Next Page Ammonium Thiosulfate Page Number: 2 Section 4. First Aid Measures Eye Contact Check for and remove any contact lenses. In case of contact, immediately flush eyes with plenty of water for at least 15 minutes. Cold water may be used. Get medical attention if irritation occurs. -

Exhibit 2D-3
Exhibit 2D–3. Hazardous Substances 1. Acetaldehyde 73. Captan 144. Ferrous sulfate 2. Acetic acid 74. Carbaryl 145. Formaldehyde 3. Acetic anhydride 75. Carbofuran 146. Formic acid 4. Acetone cyanohydrin 76. Carbon disulfide 147. Fumaric acid 5. Acetyl bromide 77. Carbon tetrachloride 148. Furfural 6. Acetyl chloride 78. Chlordane 149. Guthion 7. Acrolein 79. Chlorine 150. Heptachlor 8. Acrylonitrile 80. Chlorobenzene 151. Hexachlorocyclopentadiene 9. Adipic acid 81. Chloroform 152. Hydrochloric acid 10. Aldrin 82. Chloropyrifos 153. Hydrofluoric acid 11. Allyl alcohol 83. Chlorosulfonic acid 154. Hydrogen cyanide 12. Allyl chloride 84. Chromic acetate 155. Hydrogen sulfide 13. Aluminum sulfate 85. Chromic acid 156. Isoprene 14. Ammonia 86. Chromic sulfate 157. Isopropanolamine dodecylbenzenesulfonate 15. Ammonium acetate 87. Chromous chloride 158. Kelthane 16. Ammonium benzoate 88. Cobaltous bromide 159. Kepone 17. Ammonium bicarbonate 89. Cobaltous formate 160. Lead acetate 18. Ammonium bichromate 90. Cobaltous sulfamate 161. Lead arsenate 19. Ammonium bifluoride 91. Coumaphos 162. Lead chloride 20. Ammonium bisulfite 92. Cresol 163. Lead fluoborate 21. Ammonium carbamate 93. Crotonaldehyde 164. Lead fluorite 22. Ammonium carbonate 94. Cupric acetate 165. Lead iodide 23. Ammonium chloride 95. Cupric acetoarsenite 166. Lead nitrate 24. Ammonium chromate 96. Cupric chloride 167. Lead stearate 25. Ammonium citrate 97. Cupric nitrate 168. Lead sulfate 26. Ammonium fluoroborate 98. Cupric oxalate 169. Lead sulfide 27. Ammonium fluoride 99. Cupric sulfate 170. Lead thiocyanate 28. Ammonium hydroxide 100. Cupric sulfate ammoniated 171. Lindane 29. Ammonium oxalate 101. Cupric tartrate 172. Lithium chromate 30. Ammonium silicofluoride 102. Cyanogen chloride 173. Malathion 31. Ammonium sulfamate 103. Cyclohexane 174. Maleic acid 32. Ammonium sulfide 104. -
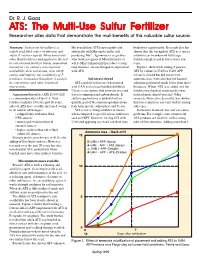
ATS: the Multi-Use Sulfur Fertilizer Researcher Cites Data That Demonstrate the Muli-Benefits of This Valuable Sulfur Source
Dr. R. J. Goos ATS: The Multi-Use Sulfur Fertilizer Researcher cites data that demonstrate the muli-benefits of this valuable sulfur source. Summary: Ammonium thiosulfate is a Mn availability. ATS reacts rapidly and hydrolysis significantly. Research also has widely used fluid source of nitrogen and abiotically with Mn-oxides in the soil, shown that the strength of ATS as a urease sulfur. It oxidizes rapidly. When mixed with producing Mn2+. Agronomists or growers inhibitor can be enhanced with large other fluid fertilizers and applied to the soil who work in regions of Mn deficiency, or fertilizer droplets and at lower water con- in concentrated fertilizer bands, ammonium where Mn fertilization helps reduce certain tents. thiosulfate can enhance micronutrient crop diseases, are encouraged to experiment Figure 1 shows how adding 5 percent availability, slow soil urease, slow nitrifi- with ATS. ATS by volume to UAN or UAN-APP cation, and improve the availability of P mixtures slowed but did not prevent fertilizers. Ammonium thiosulfate is used for Soil urease slowed ammonia loss. Note also that the banded its convenience and other beneficial ATS can slow soil urease when mixed solutions performed much better than those interactions. with UAN and surface banded (dribbled). broadcast. Where ATS was added and the Urease is an enzyme that converts urea and fertilizer was banded, ammonia loss was Ammonium thiosulfate (ATS 12-0-0-26S) water to ammonia and carbon dioxide. If reduced more than 60 percent! Other is a standard product of the U.S. fluid surface-applied urea is hydrolyzed too research, while also successful, has shown fertilizer industry.