Utilization of Wild Relatives of Wheat, Barley, Maize and Oat in Developing Abiotic and Biotic Stress Tolerant New Varieties
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Protected Areas and the Challenge of Conserving Crop Wild Relatives
PARKS 2012 Vol 18.1 PROTECTED AREAS AND THE CHALLENGE OF CONSERVING CROP WILD RELATIVES Danny Hunter1*, Nigel Maxted2, Vernon Heywood3, Shelagh Kell2 and Teresa Borelli1 * Corresponding author, [email protected] 1 Bioversity International, Rome, Italy 2 School of Biosciences, University of Birmingham, Edgbaston, Birmingham, B15 2TT, United Kingdom 3 School of Biological Sciences, University of Reading, Reading RG6 6AS, United Kingdom ABSTRACT Crop wild relatives are a critical resource for sustaining future food security. It is widely recognized that many of the world’s protected areas contain CWR diversity. Despite this, it has not yet proved possible to undertake significant actions to conserve the CWR they contain. Many challenges and obstacles need to be addressed in order to improve this situation. Recent initiatives have started to address these challenges and uncovered some key lessons. Still, the need for action is urgent and the paper concludes by drawing attention to the need for a global approach to conserving priority and threatened CWR in the wild. INTRODUCTION worth noting that the same study found breeders’ use of CWR taxa was increasing year on year, even though it Crop wild relatives (CWR) - wild plant species closely was recognized that they were still far from being related to crops to which they may contribute beneficial systematically exploited. genes - constitute an enormous reservoir of genetic variation for crop improvement and are an important Some idea of the scale of benefits may be obtained from socio-economic resource. Genes from wild plants have published estimates referring to a selected number of provided crops with resistance to many pests and crops. -

The U.S. Oats Industry (AER-573)
C. in îtates U-- '^— ^ nentof >^^ Agriculture The U.S. Economic Research Service Oats Industry Agricultural EcofKmriic Report Linwood A. Hoffman Number 573 Janet Livezey Additional copies of this report... can be purchased from the Superintendent of Documents, U.S. Government Printing Office, Washington, DC 20402. Ask for The U.S. Oats Industry (AER-573). Write to the above address for price and ordering instructioas. For faster service, call the GPO order desk at 202-783-3238 and charge your purchase to your Visa, MasterCard, Choice, or GPO Deposit Account. A 25-percent bulk discount is available on orders of 100 or more copies shipped to a single address. Please add 25 percent extra for postage for shipments to foreign addresses. Microfiche copies (Í6.50 for each report plus Í3 for processing) can be purchased from the order desk. National Technical Information Service, 5285 Port Royal Road, Springfield, VA 22161. Enclose check or money order, payable to NTIS. For faster service, call NTIS at 703-487-4650 and charge your purchase to your Visa, MasterCard, American Express, or NTIS Deposit Account. NTIS will ship rush orders within 24 hours for an extra Í10; charge your rush order by calling 800-336-4700. The Economic Research Service has no copies for free mailing. The U.S. OATS INDUSTRY, by Linwood A. Hoffman and Janet Livezey. Commodity Economics Division, Economic Research Service, U.S. Department of Agriculture. Agricultural Economic Report No. 573. ABSTRACT U.S. farmers produced about 16 percent of the total world oats production during 1980-85, down from more than 29 percent during 1960-64 when the United States was the largest producer. -

Toward Unifying Global Hotspots of Wild and Domesticated Biodiversity
plants Review Toward Unifying Global Hotspots of Wild and Domesticated Biodiversity Samuel Pironon 1,*, James S. Borrell 1, Ian Ondo 1, Ruben Douglas 1, Charlotte Phillips 2, Colin K. Khoury 3,4 , Michael B. Kantar 5 , Nathan Fumia 5 , Marybel Soto Gomez 6,7 , Juan Viruel 1 , Rafael Govaerts 1 ,Félix Forest 1 and Alexandre Antonelli 1,8 1 Royal Botanic Gardens, Kew, Richmond TW93AQ, UK; [email protected] (J.S.B.); [email protected] (I.O.); [email protected] (R.D.); [email protected] (J.V.); [email protected] (R.G.); [email protected] (F.F.); [email protected] (A.A.) 2 Royal Botanic Gardens, Kew, Wakehurst Place TW93AE, UK; [email protected] 3 International Center for Tropical Agriculture (CIAT), Cali 6713, Colombia; [email protected] 4 Department of Biology, Saint Louis University, St. Louis, MO 63103, USA 5 Department of Tropical Plant and Soil Science, University of Hawaii at Manoa, Honolulu, HI 96822, USA; [email protected] (M.B.K.); [email protected] (N.F.) 6 Department of Botany, University of British Columbia, Vancouver, BC V6T1Z4, Canada; [email protected] 7 UBC Botanical Garden and Centre for Plant Research, University of British Columbia, Vancouver, BC V6T1Z4, Canada 8 Gothenburg Global Biodiversity Centre, Department of Biological and Environmental Sciences, University of Gothenburg, 40530 Göteborg, Sweden * Correspondence: [email protected] Received: 17 July 2020; Accepted: 27 August 2020; Published: 31 August 2020 Abstract: Global biodiversity hotspots are areas containing high levels of species richness, endemism and threat. Similarly, regions of agriculturally relevant diversity have been identified where many domesticated plants and animals originated, and co-occurred with their wild ancestors and relatives. -

SRP472 3 Comparison of Rye and Triticale As Forages for Grazing
Selected Articles from 1985 Report of Agricultural Research Southeast Branch Experiment Station Agricultural Experiment Station Kansas State University 16 Comparison of Rye and Triticale as Forages for Grazing Stocker Cattle Winter annual small grains are frequently grazed during late fall and early spring in southeastern Kansas. Wheat is often the crop of choice, especially if grain production is the primary objective. If pasture is the main consideration, there are probably other small grains that will yield more forage and produce a greater quantity of beef cattle weight gain per acre than wheat. Research has been conducted at the Southeast Kansas Experiment Station to determine which winter annual small grains will result in maximum forage and beef production in a graze-out program. A study conducted in 1981-82 revealed that triticale produced nearly twice as much beef liveweight gain per acre as Newton wheat. Results from a 1982-83 study indicated that a mixture of 2/3 rye and 1/3 wheat produced over three times as much beef liveweight per acre as triticale. The following study was conducted to compare rye and triticale with respect to performance of grazing stocker cattle. Procedure: On September 19, 1983, two 5-acre fields were seeded with winter annuals. One field was seeded with 105 lb of triticale per acre and the other was seeded with 89 lb of Bonel rye per acre. At seeding time, 25-65-70 lb of N-P2O5-K2O per acre was applied and on November 14, 1984, 50 lb of N per acre was applied to each pasture. -

Plant-Based Recipes
A collection of 100% PLANT-BASED RECIPES Breakfasts and smoothies p.02 Starters p.05 Mains p.06 Sweet treats p.12 Break- fasts and smooth- ies: Overnight Oats with Shiro Miso serves 1 The miso gives these oats their pleasant ‘umami’ taste (the ‘new’ fifth taste sensation). If you need to eat breakfast away from home, make this in an empty jam jar, and give your taste buds a treat! Nutrition Information: (Approximate nutrition information per serving with fresh fruit/ berries, nuts and maple syrup) Energy (kcals) 349 Fat (g) 12.6 Saturates (g) 1.3 Carbs (g) 47.1 Sugar (g) 5.5 Fibre (g) 6.7 Protein (g) 8.5 INGREDIENTS: METHOD: ½ cup (45 g) of porridge oats ¾ cup (180 mls) of Oatly Oat Drink 1. Mix all of the ingredients except the (preferably Whole/Barista Edition) topping in a bowl (or jam jar), cover and ½ tbsp white Shiro Miso paste leave in ‘fridge for at least 3 hours, but ideally overnight. Topping - your choice from maple 2. Remove from the ‘fridge and, if you syrup, fresh fruit/berries and wish, add more oat drink, until you get roasted nuts the desired consistency. 3. Top with your chosen toppings and enjoy! 02 Avocado & Strawberry smoothie INGREDIENTS: serves 2 This creamy smoothie is quick to make 300 ml Oatly Oat Drink and refreshing at any time of day. 150 g frozen strawberries ½ ripe avocado, peeled Nutrition Information: Juice of ½ lemon (Approximate nutrition information per serving) 1 tbsp maple or agave syrup 2 tsp chia seeds Energy (kcals) 182 Fat (g) 7.8 Saturates (g) 1.1 Carbs (g) 23.9 METHOD: Sugar (g) 13.6 Fibre (g) 4.9 1. -

Torrefaction of Oat Straw to Use As Solid Biofuel, an Additive to Organic Fertilizers for Agriculture Purposes and Activated Carbon – TGA Analysis, Kinetics
E3S Web of Conferences 154, 02004 (2020) https://doi.org/10.1051/e3sconf/202015402004 ICoRES 2019 Torrefaction of oat straw to use as solid biofuel, an additive to organic fertilizers for agriculture purposes and activated carbon – TGA analysis, kinetics Szymon Szufa1, Maciej Dzikuć2 ,Łukasz Adrian3, Piotr Piersa4, Zdzisława Romanowska- Duda5, Wiktoria Lewandowska 6, Marta Marcza7, Artur Błaszczuk8, Arkadiusz Piwowar9 1 Lodz University of Technology, Faculty of Process and Environmental Engineering, Wolczanska 213, 90-924 Lodz,, Poland, [email protected] 2 University of Zielona Góra, Faculty of Economics and Management, ul. Licealna 9, 65-246 Zielona Góra, Poland, [email protected] 3 University of Kardynal Stefan Wyszyński, Faculty of Biology and Environmental Science, Dewajtis 5, 01-815 Warszawa, Poland, [email protected] 4 Lodz University of Technology, Faculty of Process and Environmental Engineering, Wolczanska 213, 90-924 Lodz,, Poland, [email protected] 5 Laboratory of Plant Ecophysiology, Faculty of Biology and Environmental Protection, University of Lodz, Banacha str. 12/16, 90-131 Łódź, Poland, [email protected] 6 University of Lodz, Chemical Faculty, Tamka 13, 91-403 Łódź, Poland, [email protected] 7 AGH University of Science and Technology, Faculty of Energy and Fuels, al. Mickiewicza 30, 30- 059 Krakow, Poland, [email protected] 8 Czestochowa University of Technology, Institute of Advanced Energy Technologies, Dabrowskiego 73, 42-200, Czestochowa, Poland, [email protected] 9 Wroclaw University of Economics, Faculty of Engineering and Economics, Komandorska 118/120 , 53-345 Wrocław, Poland, [email protected] Abstract. -

Crop Wild Relatives: Plant Conservation for Food Security
Natural England Research Report NERR037 Crop Wild Relatives: Plant conservation for food security www.naturalengland.org.uk Natural England Research Report NERR037 Crop Wild Relatives: Plant conservation for food security John Hopkins1 and Nigel Maxted2 1Natural England 2University of Birmingham Published on 25 January 2011 © Natural England copyright 2011 ISSN 1754-1956 This material is subject to Natural England copyright protection under the Copyright Designs and Patents Act 1988. Natural England copyright protected material (other than Natural England logos) may be reproduced free of charge in any format or medium for non-commercial purposes, private study, criticism, review, news reporting and for internal circulation within your organisation. This is subject to the material being reproduced accurately and not used in a misleading context. Where any of the Natural England copyright material is being republished or copied to others, the source of the material must be identified and the copyright status acknowledged. However, if you wish to use all or part of this information for commercial purposes, including publishing you will need to apply for a licence. Applications can be sent to: Publications Natural England 3rd Floor, Touthill Close, City Road Peterborough PE1 1XN Tel: 0845 600 3078 Fax: 01733 455103 Email: [email protected] Crop Wild Relatives: Plant conservation for food security i Project details This report is a review of the scientific literature relating to Crop Wild Relatives and related aspects of crop genetic diversity conservation, carried out by the authors. A summary of the findings covered by this report, as well as Natural England's views on this research, can be found within Natural England Research Information Note RIN037 – Crop Wild Relatives: Plant conservation for food security. -

Genome Size Variation in the Genus Avena
Genome Genome size variation in the genus Avena Journal: Genome Manuscript ID gen-2015-0132.R1 Manuscript Type: Article Date Submitted by the Author: 01-Dec-2015 Complete List of Authors: Yan, Honghai; Triticeae Research Institute of Sichuan Agricultural University, Martin, Sara; Agriculture and Agri-Food Canada Bekele, Wubishet; Agriculture and Agri-Food Canada Latta, Robert;Draft Dalhousie University, Department of Biology Diederichsen, Axel; Plant Gene Resources of Canada, Agriculture and Agri- Food Canada Peng, Yuanying; Triticeae Research Institute of Sichuan Agricultural University Tinker, Nicholas; Eastern Cereal and Oilseed Research Centre Keyword: Oat, flow cytometry, nucleus, polyploidy https://mc06.manuscriptcentral.com/genome-pubs Page 1 of 41 Genome Genome size variation in the genus Avena ab a a c d Honghai Yan , Sara L. Martin , Wubishet A. Bekele , Robert G. Latta , Axel Diederichsen , Yuanying Peng b, Nicholas A. Tinker a* *correspondence a Agriculture and Agri-Food Canada, 930 Carling Ave., Bldg. 50, C.E.F., Ottawa ON K1A0C6 Canada b Triticeae Research Institute, Sichuan Agricultural University, Wenjiang, Chengdu 611130, Sichuan, People’s Republic of China Draft c Department of Biology, Dalhousie University, 1355 Oxford St. Halifax NS B3H4R2 Canada d Agriculture and Agri-Food Canada, Plant Gene Resources of Canada, 107 Science Place, Saskatoon SK S7N0X2 Canada https://mc06.manuscriptcentral.com/genome-pubs Genome Page 2 of 41 Abstract Genome size is an indicator of evolutionary distance and a metric for genome characterization. Here, we report accurate estimates of genome size in 99 accessions from 26 species of Avena . We demonstrate that the average genome size of C genome diploid species (2C=10.26 pg) is 15% larger than that of A genome species (2C=8.95 pg) and that this difference likely accounts for a progression of size among tetraploid species, where AB < AC < CC (average 2C=16.76 pg, 18.60 pg, and 21.78 pg, respectively). -

Expanding the Production of Oats and Small Grains in Iowa and Minnesota Prepared by the Sustainable Food Lab and Practical Farmers of Iowa 4/27/15
Expanding the production of oats and small grains in Iowa and Minnesota Prepared by the Sustainable Food Lab and Practical Farmers of Iowa 4/27/15 Introduction Iowa is ranked 1st in the nation in corn and soybean production and Minnesota is ranked 4th in corn and 3rd in soybean production. Corn and soybeans are by far the most dominant crops in these landscapes, farmed by some of the most sophisticated farmers on some of the best soils in the world. The system optimizes for production of corn and soybeans to the exclusion of almost all other crops. The productivity of these systems and the infrastructure (support services, product development, and policy) that has grown to support these systems have been designed for efficiency in the production of these crops at the exclusion of diversity. While the system itself is a marvel, the loss of farming with a third or fourth crop in rotation has resulted in unintended consequences, including insufficient crop diversity to enhance soil quality, and a decline in the supply of small grains (oats, wheat, rye, barley) in a region that is home to some of the largest processors of small grains in the country. Commercial Rationale Supply Oats, once one the most popular crops grown in the Upper Midwest, have been declining in production for years as a system and markets have been developed to optimize the production of corn and soybeans. U.S. farmers planted 3.03 million acres of oats in 2014, down from 23.3 million in 1968. Growing corn and soy instead of oats is also contributing to a decline in oat production in Canada, one of the world’s largest net exporters of oats, and a key source of supply for the US. -

Article on Genetic Markers for Bunt Resistance From
Let’s make grain great again Click here to sign up for the newsletter The Landrace Newsletter no. 5 May 2021 A new growing season is ahead of us, and I greet the spring with news from both future and past from the organic grain sector. I wish you joyfull reading Anders Borgen Content in this newsletter Open field day and general assembly in Landsorten, Tuesday 22. June..............................................2 But now then, is it Landsorten or Agrologica, selling organic seed in future?...................................2 Mobile stone mill for local production................................................................................................3 Nordic grain festival 28th-30th October 2021 in Norway.....................................................................4 News from Agrologica science lab......................................................................................................4 Genetic markers for bunt resistance - news from LIVESEED-, Økosort-II and bunt projects......4 Acid rain and gluten-index..............................................................................................................4 Zanduri, Macha, and the hailstorm in Georgia...............................................................................6 Colchic emmer (Triticum paleochochicum)...............................................................................6 Emmer........................................................................................................................................7 Durum........................................................................................................................................7 -
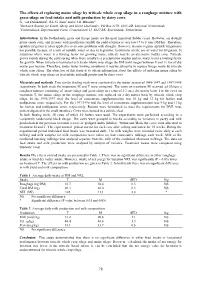
78 the Effects of Replacing Maize Silage by Triticale Whole Crop Silage
The effects of replacing maize silage by triticale whole crop silage in a roughage mixture with grass silage on feed intake and milk production by dairy cows G. van Duinkerken1, R.L.G. Zom1 and E.J.B. Bleumer2 1Research Station for Cattle, Sheep and Horse Husbandry, PO Box 2176, 8203 AD, Lelystad, Netherlands 2Cranendonck, Experimental Farm, Cranendonck 11, 6027 RK, Soerendonk, Netherlands Introduction In the Netherlands, grass and forage maize are the most important fodder crops. However, on drought prone sandy soils, and in years with insufficient rainfall the yield of maize is very low (7 to 8 tons DM/ha). Therefore, sprinkle irrigation is often applied to overcome problems with drought. However, in some regions sprinkle irrigation is not possible because of a lack of suitable water or due to legislative restrictions on the use of water for irrigation. In situations where water is a limiting factor for growing maize, triticale may be an alternative fodder crop. Triticale grows mainly during the early spring when there usually is a precipitation surplus and so, water is not a limiting factor for growth. When triticale is harvested as triticale whole crop silage the DM yield ranges between 9 and 11 ton of dry matter per hectare. Therefore, under water limiting conditions it may be attractive to replace forage maize by triticale whole crop silage. The objective of this study is to obtain information about the effects of replacing maize silage by triticale whole crop silage on feed intake and milk production by dairy cows Materials and methods Two similar feeding trials were conducted in the winter season of 1996/1997 and 1997/1998 respectively. -

Feeding Rye Or Triticale to Dairy Cattle
Feeding Rye or Triticale to Dairy Cattle Liz Binversie | Agriculture Educator | Extension Brown County Matt Akins | Assistant Scientist and Extension Specialist | Marshfield Agriculture Research Station Kevin Shelley |Extension NPM Specialist | UW-Madison Dept. of Horticulture Randy Shaver | Emeritis professor | UW-Madison Dept. of Dairy Science Introduction Use of cereal grain forages, such as rye and triticale gains. However, some farms may not raise (hybrid of rye and wheat), has become an increasingly youngstock on site and will need to consider if they important topic, and is especially relevant during should incorporate cereal forages in the dry or years when feed inventory is short. However, it is not lactating cow diets, and if so, how much to feed. The without challenges. Timing can conflict with higher farm should assess its current feed inventory to priority tasks on the farm such as alfalfa harvest and determine cereal forage needs, which may be helpful corn planting in spring, and corn silage harvest and in determining the acreage that should be planted manure application in the fall. Therefore establishing and harvested. Estimated yield is about 1 to 2 tons of and harvesting rye or triticale can be a challenge. dry matter per acre, if harvested at boot stage for Achieving optimal quality for lactating cows can be optimal quality to feed to lactating cows. Some farms another issue because quality declines can happen may only want to grow enough to feed for part of the quickly. Farmers, and their nutritionists, have shared year, while others may prefer to have enough ryelage that they need data and guidance to make good inventory to feed all year round.