Origin, Differentiation, and Function of Intestinal Macrophages
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
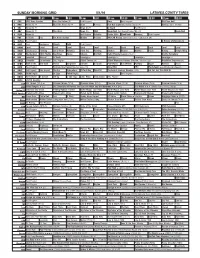
Sunday Morning Grid 5/1/16 Latimes.Com/Tv Times
SUNDAY MORNING GRID 5/1/16 LATIMES.COM/TV TIMES 7 am 7:30 8 am 8:30 9 am 9:30 10 am 10:30 11 am 11:30 12 pm 12:30 2 CBS CBS News Sunday Face the Nation (N) Paid Program Boss Paid Program PGA Tour Golf 4 NBC News (N) Å Meet the Press (N) Å News Rescue Red Bull Signature Series (Taped) Å Hockey: Blues at Stars 5 CW News (N) Å News (N) Å In Touch Paid Program 7 ABC News (N) Å This Week News (N) NBA Basketball First Round: Teams TBA. (N) Basketball 9 KCAL News (N) Joel Osteen Schuller Pastor Mike Woodlands Amazing Paid Program 11 FOX In Touch Paid Fox News Sunday Midday Prerace NASCAR Racing Sprint Cup Series: GEICO 500. (N) 13 MyNet Paid Program A History of Violence (R) 18 KSCI Paid Hormones Church Faith Paid Program 22 KWHY Local Local Local Local Local Local Local Local Local Local Local Local 24 KVCR Landscapes Painting Joy of Paint Wyland’s Paint This Painting Kitchen Mexico Martha Pépin Baking Simply Ming 28 KCET Wunderkind 1001 Nights Bug Bites Space Edisons Biz Kid$ Celtic Thunder Legacy (TVG) Å Soulful Symphony 30 ION Jeremiah Youssef In Touch Leverage Å Leverage Å Leverage Å Leverage Å 34 KMEX Conexión En contacto Paid Program Fútbol Central (N) Fútbol Mexicano Primera División: Toluca vs Azul República Deportiva (N) 40 KTBN Walk in the Win Walk Prince Carpenter Schuller In Touch PowerPoint It Is Written Pathway Super Kelinda Jesse 46 KFTR Paid Program Formula One Racing Russian Grand Prix. -

Cancer Forum
March 2006 Volume 30 Number 1 ISSN 0311-306X CANCER FORUM Contents nnn Forum: Psycho-oncology Overview 3 Jane Turner Psychosocial aspects of sexuality and fertility after a diagnosis of breast cancer 6 Belinda Thewes and Kate White The effect of adjuvant chemotherapy on cognitive functioning in early breast cancer: 10 implications for outcomes research and oncology practice Geoffrey F Beadle et al. Exercise in cancer recovery: an overview of the evidence 13 Sandra C Hayes and Beth Newman Psychosocial issues for people with advanced cancer: overcoming the research challenges 18 Penelope Schofield et al. Challenges experienced by informal caregivers in cancer 21 Afaf Girgis et al. Leading the way – best practice in psychosocial care for cancer patients 25 Karen Luxford and Jane Fletcher Translating psychosocial care: guidelines into action 28 Suzanne K Steginga et al. The Psycho-oncology Co-operative Research Group 32 Phyllis Butow and Rebecca Hagerty nnn Articles Diversity and availability of support groups 35 Laura Kirsten et al. Promoting shared decision making and informed choice for the early detection of prostate 38 cancer: development and evaluation of a GP education program Robyn Metcalfe et al. nnn Reports Support for research 43 Australian behavioural research in cancer 57 COSA Annual Scientific Meeting 2005 65 Crossing the boundaries: a new era in cancer consumer participation 65 nnn News and announcements 67 nnn Book reviews 70 nnn Calendar of meetings 81 CancerForum Volume 30 Number 1 March 2006 1 CANCER FORUM FORUM Psycho-oncology OVERVIEW Jane Turner n Department of Psychiatry, University of Queensland Email: [email protected] “I stared down her housedress as she bent over to bathe me. -

El Llegat Dels Germans Grimm En El Segle Xxi: Del Paper a La Pantalla Emili Samper Prunera Universitat Rovira I Virgili [email protected]
El llegat dels germans Grimm en el segle xxi: del paper a la pantalla Emili Samper Prunera Universitat Rovira i Virgili [email protected] Resum Les rondalles que els germans Grimm van recollir als Kinder- und Hausmärchen han traspassat la frontera del paper amb nombroses adaptacions literàries, cinema- togràfiques o televisives. La pel·lícula The brothers Grimm (2005), de Terry Gilli- am, i la primera temporada de la sèrie Grimm (2011-2012), de la cadena NBC, són dos mostres recents d’obres audiovisuals que han agafat les rondalles dels Grimm com a base per elaborar la seva ficció. En aquest article s’analitza el tractament de les rondalles que apareixen en totes dues obres (tenint en compte un precedent de 1962, The wonderful world of the Brothers Grimm), així com el rol que adopten els mateixos germans Grimm, que passen de creadors a convertir-se ells mateixos en personatges de ficció. Es recorre, d’aquesta manera, el camí invers al que han realitzat els responsables d’aquestes adaptacions: de la pantalla (gran o petita) es torna al paper, mostrant quines són les rondalles dels Grimm que s’han adaptat i de quina manera s’ha dut a terme aquesta adaptació. Paraules clau Grimm, Kinder- und Hausmärchen, The brothers Grimm, Terry Gilliam, rondalla Summary The tales that the Grimm brothers collected in their Kinder- und Hausmärchen have gone beyond the confines of paper with numerous literary, cinematographic and TV adaptations. The film The Brothers Grimm (2005), by Terry Gilliam, and the first season of the series Grimm (2011–2012), produced by the NBC network, are two recent examples of audiovisual productions that have taken the Grimm brothers’ tales as a base on which to create their fiction. -

TLEX GRID (EAST REGULAR) - APRIL 2021 (4/12/2021 - 4/18/2021) - WEEK #16 Date Updated:3/25/2021 2:29:43 PM
TLEX GRID (EAST REGULAR) - APRIL 2021 (4/12/2021 - 4/18/2021) - WEEK #16 Date Updated:3/25/2021 2:29:43 PM MON (4/12/2021) TUE (4/13/2021) WED (4/14/2021) THU (4/15/2021) FRI (4/16/2021) SAT (4/17/2021) SUN (4/18/2021) SHOP LC (PAID PROGRAM SHOP LC (PAID PROGRAM SHOP LC (PAID PROGRAM SHOP LC (PAID PROGRAM SHOP LC (PAID PROGRAM SHOP LC (PAID PROGRAM SHOP LC (PAID PROGRAM 05:00A 05:00A NETWORK) NETWORK) NETWORK) NETWORK) NETWORK) NETWORK) NETWORK) PAID PROGRAM PAID PROGRAM PAID PROGRAM PAID PROGRAM PAID PROGRAM PAID PROGRAM PAID PROGRAM 05:30A 05:30A (NETWORK) (NETWORK) (NETWORK) (NETWORK) (NETWORK) (NETWORK) (NETWORK) PAID PROGRAM PAID PROGRAM PAID PROGRAM PAID PROGRAM PAID PROGRAM PAID PROGRAM PAID PROGRAM 06:00A 06:00A (NETWORK) (NETWORK) (NETWORK) (NETWORK) (NETWORK) (NETWORK) (NETWORK) PAID PROGRAM PAID PROGRAM PAID PROGRAM PAID PROGRAM PAID PROGRAM PAID PROGRAM PAID PROGRAM 06:30A 06:30A (SUBNETWORK) (SUBNETWORK) (SUBNETWORK) (SUBNETWORK) (SUBNETWORK) (NETWORK) (NETWORK) PAID PROGRAM PAID PROGRAM PAID PROGRAM PAID PROGRAM PAID PROGRAM PAID PROGRAM PAID PROGRAM 07:00A 07:00A (NETWORK) (NETWORK) (NETWORK) (NETWORK) (NETWORK) (NETWORK) (SUBNETWORK) PAID PROGRAM PAID PROGRAM PAID PROGRAM PAID PROGRAM PAID PROGRAM PAID PROGRAM PAID PROGRAM 07:30A 07:30A (NETWORK) (NETWORK) (NETWORK) (NETWORK) (NETWORK) (NETWORK) (SUBNETWORK) PAID PROGRAM PAID PROGRAM PAID PROGRAM PAID PROGRAM PAID PROGRAM PAID PROGRAM PAID PROGRAM 08:00A 08:00A (NETWORK) (NETWORK) (NETWORK) (NETWORK) (NETWORK) (NETWORK) (NETWORK) CASO CERRADO CASO CERRADO CASO CERRADO -

On the Role of Chromosomal Rearrangements in Evolution
On the role of chromosomal rearrangements in evolution: Reconstruction of genome reshuffling in rodents and analysis of Robertsonian fusions in a house mouse chromosomal polymorphism zone by Laia Capilla Pérez A thesis submitted for the degree of Doctor of Philosophy in Animal Biology Supervisors: Dra. Aurora Ruiz-Herrera Moreno and Dr. Jacint Ventura Queija Institut de Biotecnologia i Biomedicina (IBB) Departament de Biologia Cel·lular, Fisiologia i Immunologia Departament de Biologia Animal, Biologia Vegetal i Ecologia Universitat Autònoma de Barcelona Supervisor Supervisor PhD candidate Aurora Ruiz-Herrera Moreno Jacint Ventura Queija Laia Capilla Pérez Bellaterra, 2015 A la mare Al pare Al mano “Visto a la luz de la evolución, la biología es, quizás, la ciencia más satisfactoria e inspiradora. Sin esa luz, se convierte en un montón de hechos varios, algunos de ellos interesantes o curiosos, pero sin formar ninguna visión conjunta.” Theodosius Dobzhansky “La evolución es tan creativa. Por eso tenemos jirafas.” Kurt Vonnegut This thesis was supported by grants from: • Ministerio de Economía y Competitividad (CGL2010-15243 and CGL2010- 20170). • Generalitat de Catalunya, GRQ 1057. • Ministerio de Economía y Competitividad. Beca de Formación de Personal Investigador (FPI) (BES-2011-047722). • Ministerio de Economía y Competitividad. Beca para la realización de estancias breves (EEBB-2011-07350). Covers designed by cintamontserrat.blogspot.com INDEX Abstract 15-17 Acronyms 19-20 1. GENERAL INTRODUCTION 21-60 1.1 Chromosomal rearrangements -

Kinsella Feb 13
MORPHEUS: A BILDUNGSROMAN A PARTIALLY BACK-ENGINEERED AND RECONSTRUCTED NOVEL MORPHEUS: A BILDUNGSROMAN A PARTIALLY BACK-ENGINEERED AND RECONSTRUCTED NOVEL JOHN KINSELLA B L A Z E V O X [ B O O K S ] Buffalo, New York Morpheus: a Bildungsroman by John Kinsella Copyright © 2013 Published by BlazeVOX [books] All rights reserved. No part of this book may be reproduced without the publisher’s written permission, except for brief quotations in reviews. Printed in the United States of America Interior design and typesetting by Geoffrey Gatza First Edition ISBN: 978-1-60964-125-2 Library of Congress Control Number: 2012950114 BlazeVOX [books] 131 Euclid Ave Kenmore, NY 14217 [email protected] publisher of weird little books BlazeVOX [ books ] blazevox.org 21 20 19 18 17 16 15 14 13 12 01 02 03 04 05 06 07 08 09 10 B l a z e V O X trip, trip to a dream dragon hide your wings in a ghost tower sails crackling at ev’ry plate we break cracked by scattered needles from Syd Barrett’s “Octopus” Table of Contents Introduction: Forging the Unimaginable: The Paradoxes of Morpheus by Nicholas Birns ........................................................ 11 Author’s Preface to Morpheus: a Bildungsroman ...................................................... 19 Pre-Paradigm .................................................................................................. 27 from Metamorphosis Book XI (lines 592-676); Ovid ......................................... 31 Building, Night ...................................................................................................... -

MAY 2021 (4/26/2021 - 5/2/2021) - WEEK #18 Date Updated:4/16/2021 2:24:45 PM
TLEX GRID (EAST REGULAR) - MAY 2021 (4/26/2021 - 5/2/2021) - WEEK #18 Date Updated:4/16/2021 2:24:45 PM MON (4/26/2021) TUE (4/27/2021) WED (4/28/2021) THU (4/29/2021) FRI (4/30/2021) SAT (5/1/2021) SUN (5/2/2021) SHOP LC (PAID PROGRAM SHOP LC (PAID PROGRAM SHOP LC (PAID PROGRAM SHOP LC (PAID PROGRAM SHOP LC (PAID PROGRAM SHOP LC (PAID PROGRAM SHOP LC (PAID PROGRAM 05:00A 05:00A NETWORK) NETWORK) NETWORK) NETWORK) NETWORK) NETWORK) NETWORK) PAID PROGRAM PAID PROGRAM PAID PROGRAM PAID PROGRAM PAID PROGRAM PAID PROGRAM PAID PROGRAM 05:30A 05:30A (NETWORK) (NETWORK) (NETWORK) (NETWORK) (NETWORK) (NETWORK) (NETWORK) PAID PROGRAM PAID PROGRAM PAID PROGRAM PAID PROGRAM PAID PROGRAM PAID PROGRAM PAID PROGRAM 06:00A 06:00A (NETWORK) (NETWORK) (NETWORK) (NETWORK) (NETWORK) (NETWORK) (NETWORK) PAID PROGRAM PAID PROGRAM PAID PROGRAM PAID PROGRAM PAID PROGRAM PAID PROGRAM PAID PROGRAM 06:30A 06:30A (SUBNETWORK) (SUBNETWORK) (SUBNETWORK) (SUBNETWORK) (SUBNETWORK) (NETWORK) (NETWORK) PAID PROGRAM PAID PROGRAM PAID PROGRAM PAID PROGRAM PAID PROGRAM PAID PROGRAM PAID PROGRAM 07:00A 07:00A (NETWORK) (NETWORK) (NETWORK) (NETWORK) (NETWORK) (NETWORK) (SUBNETWORK) PAID PROGRAM PAID PROGRAM PAID PROGRAM PAID PROGRAM PAID PROGRAM PAID PROGRAM PAID PROGRAM 07:30A 07:30A (NETWORK) (NETWORK) (NETWORK) (NETWORK) (NETWORK) (NETWORK) (SUBNETWORK) PAID PROGRAM PAID PROGRAM PAID PROGRAM PAID PROGRAM PAID PROGRAM PAID PROGRAM PAID PROGRAM 08:00A 08:00A (NETWORK) (NETWORK) (NETWORK) (NETWORK) (NETWORK) (NETWORK) (NETWORK) CASO CERRADO CASO CERRADO CASO CERRADO CASO -

The Werewolf in Lore and Legend Plate I
Montague Summers The Werewolf IN Lore and Legend Plate I THE WARLOCKERS’ METAMORPHOSIS By Goya THE WEREWOLF In Lore and Legend Montague Summers Intrabunt lupi rapaces in uos, non parcentes gregi. Actus Apostolorum, XX, 29. DOVER PUBLICATIONS, INC. Mineola New York Bibliographical Note The Werewolf in Lore and Legend, first published in 2003, is an unabridged republication of the work originally published in 1933 by Kegan Paul, Trench, Trubner & Co., Ltd., London, under the title The Werewolf. Library ofCongress Cataloging-in-Publication Data Summers, Montague, 1880-1948. [Werewolf] The werewolf in lore and legend / Montague Summers, p. cm. Originally published: The werewolf. London : K. Paul, Trench, Trubner, 1933. Includes bibliographical references and index. ISBN 0-486-43090-1 (pbk.) 1. Werewolves. I. Title. GR830.W4S8 2003 398'.45—dc22 2003063519 Manufactured in the United States of America Dover Publications, Inc., 3 1 East 2nd Street, Mineola, N.Y. 11501 CONTENTS I. The Werewolf: Lycanthropy II. The Werewolf: His Science and Practice III. The Werewolf in Greece and Italy, Spain and Portugal IV. The Werewolf in England and Wales, Scotland and Ireland V. The Werewolf in France VI. The Werewolf in the North, in Russia and Germany A Note on the Werewolf in Literature Bibliography Witch Ointments. By Dr. H. J. Norman Index LIST OF ILLUSTRATIONS I. The Warlocks’ Metamorphosis. By Goya. Formerly in the Collection of the Duke d’Osuna II. A Werewolf Attacks a Man. From Die Emeis of Johann Geiler von Kaisersberg III. The Transvection of Witches. From Ulrich Molitor’s De Lamiis IV. The Wild Beast of the Gevaudan. -
Legislature Seeking TVA Negotiation
FRIDAY 160th yEar • no. 299 aPriL 17, 2015 cLEVELand, Tn 22 PaGES • 50¢ Bulletin Legislature seeking TVA negotiation Special Olympics postponed due to wet conditions Ocoee River’s whitewater rafting future is targeted According to the document, the U.S. tination.” From Staff Reports By BRIAN GRAVES Banner Staff Writer Congress passed legislation in 1983 to provide “By passing SJR166 unanimously, the Due to wet field conditions “recreational water releases” from the Ocoee Tennessee General Assembly recognizes the in Benny Monroe Stadium, The Tennessee General Assembly has unan- No. 2 power project and enabled a contract vital impact the Ocoee River and the rafting the 33rd annual Special imously passed a joint resolution asking action between the state and TVA to provide reliable industry has on the economy of Polk County Olympics has been post- by TVA, the state and the U.S. Congress to be releases for 116 days per year. and Southeast Tennessee,” Bell told the poned to Saturday, April 25. taken to maintain “reliable water releases” That contract will expire in March 2019 with Cleveland Daily Banner. “I hope the TVA board Starting time will remain 9 along the Ocoee River. the last recreational release for rafting occur- of directors and our congressional delegation a.m. State Sen. Mike Bell, R-Riceville, introduced ring in October 2018. realize how important settling the water The popular annual event the measure and state Rep. Dan Howell, R- The resolution says the Legislature supports release issue is to our rafting businesses and Georgetown, is expressing concern the agency actions that will maintain the Ocoee’s status was supposed to have been Howell held Saturday (tomorrow). -
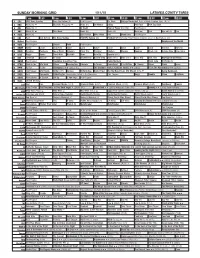
Sunday Morning Grid 1/11/15 Latimes.Com/Tv Times
SUNDAY MORNING GRID 1/11/15 LATIMES.COM/TV TIMES 7 am 7:30 8 am 8:30 9 am 9:30 10 am 10:30 11 am 11:30 12 pm 12:30 2 CBS CBS News Sunday Face the Nation (N) Paid Program Dr. Chris College Basketball Duke at North Carolina State. (N) Å 4 NBC News (N) Å Meet the Press (N) Å News (N) On Money Skiing PGA Tour PGA Tour Golf 5 CW News (N) Å In Touch Hour of Power (N) (TVG) Paid Program 7 ABC News (N) Å This Week News (N) News (N) News Å Paid Eye on L.A. Paid 9 KCAL News (N) Joel Osteen Mike Webb Paid Woodlands Paid Program 11 FOX Paid Joel Osteen Fox News Sunday FOX NFL Sunday (N) Football NFC Divisional Playoff Dallas Cowboys at Green Bay Packers. (N) 13 MyNet Paid Program Employee of the Month 18 KSCI Paid Program Church Faith Paid Program 22 KWHY Como Local Jesucristo Local Local Gebel Local Local Local Local Transfor. Transfor. 24 KVCR Painting Dewberry Joy of Paint Wyland’s Paint This Painting Kitchen Mexico Cooking Chefs Life Simply Ming Ciao Italia 28 KCET Raggs Space Travel-Kids Biz Kid$ News Asia Biz Special (TVG) 30 ION Jeremiah Youssef In Touch Bucket-Dino Bucket-Dino Doki (TVY7) Doki (TVY7) Dive, Olly Dive, Olly Revenge of the Nerds 34 KMEX Paid Program República Deportiva (TVG) Pablo Montero Hotel Todo Al Punto (N) 40 KTBN Walk in the Win Walk Prince Redemption Liberate In Touch PowerPoint It Is Written B. -

Proquest Dissertations
NOTE TO USERS This reproduction is the best copy available. UMf University of Alberta Cultural Contexts and Cultural Change: The Werewolf in Classical, Medieval, and Modern Texts by Renee Michelle Ward A thesis submitted to the Faculty of Graduate Studies and Research in partial fulfillment of the requirements for the degree of Doctor of Philosophy in English Department of English and Film Studies ©Renee Michelle Ward Spring 2009 Edmonton, Alberta Permission is hereby granted to the University of Alberta Libraries to reproduce single copies of this thesis and to lend or sell such copies for private, scholarly or scientific research purposes only. Where the thesis is converted to, or otherwise made available in digital form, the University of Alberta will advise potential users of the thesis of these terms. The author reserves all other publication and other rights in association with the copyright in the thesis and, except as herein before provided, neither the thesis nor any substantial portion thereof may be printed or otherwise reproduced in any material form whatsoever without the author's prior written permission. Library and Archives Bibliotheque et 1*1 Canada Archives Canada Published Heritage Direction du Branch Patrimoine de I'edition 395 Wellington Street 395, rue Wellington Ottawa ON K1A 0N4 OttawaONK1A0N4 Canada Canada Your file Votre reference ISBN: 978-0-494-55632-0 Our file Notre reference ISBN: 978-0-494-55632-0 NOTICE: AVIS: The author has granted a non L'auteur a accorde une licence non exclusive exclusive license allowing -

+ 14 Days of Tv Listings Free
CINEMA VOD NETFLIX SPORTS TECH + 14 DAYS OF TV LISTINGS MAY 2015 ISSUE 1 TVGUIDE.CO.UK TVDAILY.COM Mad Max Doctor Who Better Call Saul Football Plex FREE MAY 2015 Issue 1 Contents TVGUIDE.CO.UK TVDAILY.COM EDITOR’S 4 Latest TV News Fans Bring LETTER 14 Fans are the driving force The biggest news stories from the behind any popular show. world of television. They engage with the Fantasy To Life content, they talk to their friends, and they drive up Game of Thrones fans recreate the the ratings. They create world of Westeros. works of art, write epic- length fanfiction, and even produce their own films! Check out the astonishing efforts of Game of Thrones 17 Food fans on page 14 if you don’t believe us. We’d like Your television dinners sorted with to dedicate our inaugural inspiration from our favourite sitcoms. issue to the fans who make everything we do possible. If you picked up this magazine because 6 Top 100 WTF you love television, give 18 Football yourself a pat on the back All you need to know about the final – you’re the best. Moments (Part 1) games of the season and how to cope Susan Brett, Editor Some of the most unbelievable twists with post-season blues. TVGuide.co.uk ever to grace the small screen. Part two 104-08 Oxford Street, London, W1D 1LP next issue. [email protected] 22 Install A Home Media CONTENT Editor: Susan Brett Deputy Editor: Ally Russell Big Screen Guide Server Designer: Francisco Torres 8 Everything you need to know about An easy-to-understand guide to FOR ADVERTISING ENQUIRIES Head of Cross-Platform: what’s new and coming soon to the installing and sharing content on the Andrew Webb 020-3056-1802 Box Office.