Unit 1 – Lesson 2 Relating Metric and Imperial Systems
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

In Physical Chemistry
INTERNATIONAL UNION OF PURE AND APPLIED CHEMISTRY Physical and Biophysical Chemistry Division IUPAC Vc^llclllul LICb . U 111 Lb cillCl Oy 111 DOlo in Physical Chemistry Third Edition Prepared for publication by E. Richard Cohen Tomislav Cvitas Jeremy G. Frey BcTt.il Holmstrom Kozo Kuchitsu R,oberto Marquardt Ian Mills Franco Pavese Martin Quack Jiirgen Stohner Herbert L. Strauss Michio Takanii Anders J Thor The first and second editions were prepared for publication by Ian Mills Tomislav Cvitas Klaus Homann Nikola Kallay Kozo Kuchitsu IUPAC 2007 RSC Publishing CONTENTS v PREFACE ix HISTORICAL INTRODUCTION xi 1 PHYSICAL QUANTITIES AND UNITS 1 1.1 Physical quantities and quantity calculus 3 1.2 Base quantities and derived quantities 4 1.3 Symbols for physical quantities and units 5 1.3.1 General rules for symbols for quantities 5 1.3.2 General rules for symbols for units 5 1.4 Use of the words "extensive", "intensive", "specific", and "molar" 6 1.5 Products and quotients of physical quantities and units 7 1.6 The use of italic and Roman fonts for symbols in scientific publications 7 2 TABLES OF PHYSICAL QUANTITIES 11 2.1 Space and time 13 2.2 Classical mechanics 14 2.3 Electricity and magnetism 16 2.4 Quantum mechanics and quantum chemistry 18 2.4.1 Ab initio Hartree-Fock self-consistent field theory (ab initio SCF) 20 2.4.2 Hartree-Fock-Roothaan SCF theory, using molecular orbitals expanded as linear combinations of atomic-orbital basis . functions (LCAO-MO theory) . 21 2.5 Atoms and molecules 22 2.6 Spectroscopy 25 2.6.1 Symbols for angular -
![Homework 1: Exercise 1 Metric - English Conversion [Based on the Chauffe & Jefferies (2007)]](https://docslib.b-cdn.net/cover/5363/homework-1-exercise-1-metric-english-conversion-based-on-the-chauffe-jefferies-2007-975363.webp)
Homework 1: Exercise 1 Metric - English Conversion [Based on the Chauffe & Jefferies (2007)]
MAR 110 HW 1: Exercise 1 – Conversions p. 1 Homework 1: Exercise 1 Metric - English Conversion [based on the Chauffe & Jefferies (2007)] 1-1. THE METRIC SYSTEM The French developed the metric system during the 1790s. Unlike the "English" system, in which the difference between smaller and larger units is irregular and has no scientific foundation, the metric system is based on multiples of 10. Smaller and larger units can be obtained simply by moving the decimal point - that is multiplying or dividing by 10. For example, in the "English" system there are 12 inches to a foot (ft), but 3 ft to a yard, and 1760 yards in a mile. By contrast, in the metric system there are 10 m to a decameter (dm), 10 dm to a hectometer (hm), and 10 hm to a kilometer (km). The convenience and regularity of the metric system make it the standard system used in scientific research The basic units of the metric system are the meter for length, gram for mass (weight), liter for volume, and degree Celsius (OC) for temperature. These terms have been defined in terms of practical considerations. For example, one meter is equal to one ten millionth of the distance between the North Pole and the Equator. The gram is the mass of one cubic centimeter (or one millionth of a cubic meter) of water at a temperature of 4°C. The liter is the volume of a cubic decimeter (or one thousandth of a cubic meter). A degree Celsius is one-hundredth of the change in temperature between the freezing and boiling points of water. -

Unit Conversion
Unit Conversion Subject Area(s) Mathematics and Engineering Associated Unit: Units of Length Activity Title: Unit Conversion Image 1: 2 [position: centered] Figure 1: Units of Measurements Image 1 ADA Description: Figure 1 above shows different units of measurements, this image highlights different measurement that are used in everyday life Caption: Units of Measurements Image file: Figure_1 Source/Rights: http://www.dimensionsguide.com/unit-conversion/ Grade Level _4_ (_3_-_4_) Activity Dependency Time Required 45 Group Size 3-5 Expendable Cost per Group US $0 Summary In this activity students will gain a better understanding of different units of measurements. Students fail to grasp the concept of different units of measurements and conversion at an early stage in their education. The problems that students are asked to solve in classroom such as converting from either yard to feet or feet to inches and so forth may seem simple to us adults and teachers, however to students the arithmetic may be difficult and often the concept of “unit conversion” may be abstract. Although Students may know the conversion factors such as 1 feet is equivalent to 12 inches or 1 yard is equal to 3 feet, they have not yet developed a mental model of the magnitude these units represent (i.e. inches make up feet therefore a foot is larger in magnitude than inches). The goal of this activity is to develop and strengthen the mental representation of different units so that students may convert one unit to another with ease. Furthermore, this activity will also give students a visual and physical representation of the magnitude of feet, yard and inches are. -

Not Made to Measure
Made in Britain: Not made to measure. Ronnie Cohen © 2011 Ronnie Cohen. All rights reserved. 1 Table of Contents Foreword...............................................................................................................................................5 Introduction..........................................................................................................................................6 Central Role of Measurement in Daily Life.........................................................................................7 Why Measurement Matters..................................................................................................................8 Quest for Honest Measurements since Ancient Times.........................................................................9 Measurement Facts: Did you know that....?.......................................................................................10 Description of the British Imperial System........................................................................................11 Introduction to the British Imperial System..............................................................................11 Units of Length..........................................................................................................................11 Units of Area.............................................................................................................................11 Units of Volume........................................................................................................................12 -
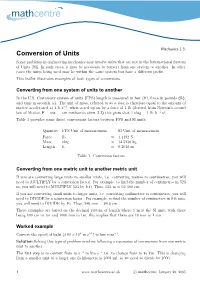
Conversion of Units Some Problems in Engineering Mechanics May Involve Units That Are Not in the International System of Units (SI)
Mechanics 1.3. Conversion of Units Some problems in engineering mechanics may involve units that are not in the International System of Units (SI). In such cases, it may be necessary to convert from one system to another. In other cases the units being used may be within the same system but have a different prefix. This leaflet illustrates examples of both types of conversions. Converting from one system of units to another In the U.S. Customary system of units (FPS) length is measured in feet (ft), force in pounds (lb), and time in seconds (s). The unit of mass, referred to as a slug, is therefore equal to the amount of − matter accelerated at 1 ft s 2, when acted up on by a force of 1 lb (derived from Newton’s second law of Motion F = ma — see mechanics sheet 2.2) this gives that 1 slug = 1 lb ft−1 s2. Table 1 provides some direct conversions factors between FPS and SI units. Quantity FPS Unit of measurement SI Unit of measurement Force lb = 4.4482 N Mass slug = 14.5938 kg Length ft =0.3048m Table 1: Conversion factors Converting from one metric unit to another metric unit If you are converting large units to smaller units, i.e. converting metres to centimetres, you will need to MULTIPLY by a conversion factor. For example, to find the number of centimetres in 523 m, you will need to MULTIPLY 523 by 100. Thus, 523 m = 52 300 cm. If you are converting small units to larger units, i.e. -

Physics and Measurement
PP UZZLERUZZLER For thousands of years the spinning Earth provided a natural standard for our measurements of time. However, since 1972 we have added more than 20 “leap seconds” to our clocks to keep them synchronized to the Earth. Why are such adjustments needed? What does it take to be a good standard? (Don Mason/The Stock Market and NASA) chapter Physics and Measurement Chapter Outline 1.1 Standards of Length, Mass, and 1.5 Conversion of Units Time 1.6 Estimates and Order-of-Magnitude 1.2 The Building Blocks of Matter Calculations 1.3 Density 1.7 Significant Figures 1.4 Dimensional Analysis 2 ike all other sciences, physics is based on experimental observations and quan- titative measurements. The main objective of physics is to find the limited num- Lber of fundamental laws that govern natural phenomena and to use them to develop theories that can predict the results of future experiments. The funda- mental laws used in developing theories are expressed in the language of mathe- matics, the tool that provides a bridge between theory and experiment. When a discrepancy between theory and experiment arises, new theories must be formulated to remove the discrepancy. Many times a theory is satisfactory only under limited conditions; a more general theory might be satisfactory without such limitations. For example, the laws of motion discovered by Isaac Newton (1642–1727) in the 17th century accurately describe the motion of bodies at nor- mal speeds but do not apply to objects moving at speeds comparable with the speed of light. In contrast, the special theory of relativity developed by Albert Ein- stein (1879–1955) in the early 1900s gives the same results as Newton’s laws at low speeds but also correctly describes motion at speeds approaching the speed of light. -

Teaching Guides; Units of Study (Subject Fields) IDENTIFIERS ESEA Title III
DOCUMENT RESUME ED 069 524 SE 015 335 AUTHOR Cox, Philip L. TITLE EXploring Linear Measure, Teacher's Guide. INSTITUTION Oakland County Schools, Pontiac, Mich. SPONS AGENCY Bureau of Elementary and Secondary Education (DREW /OE), Washington, D.C. PUB DATE Oct 69 GRANT OEG-68-05635-0 NOTE 226p.; Revised Edition EDRS PRICE MF-$0.65 HC-$9.87 DESCRIPTORS Curriculum; Instruction; *Instructional Materials; Low Ability Students; Mathematics Education; *Measurement; Metric System; Objectives; *Secondary School Mathematics; *Teaching Guides; Units of Study (Subject Fields) IDENTIFIERS ESEA Title III ABSTRACT This guide to accompany "Exploring Linear Measure," contains all of the student materials in SE 015 334 plus supplemental teacher materials. It includes a listing of terminal objectives, necessary equipment and teaching aids, and resource materials. Answers are given to all problems and suggestions and activities are presented for each section. Related documents are SE 015 334 and SE 015 336 through SE 015 347. This work was prepared under an ESEA Title III contract.(LS) U S Ill PAH l%iis. Co tots'ot Ios ()LICA Ilo% S %I I f 41(f O F ICI Of t DuCst 110h FILMED FROM BEST AVAILABLE COPY 4, sl sos ; EXPLORING LINEAR MEASURE ITY 11 12 13 14 15 16. 19 20 21 22 TENTHS OF ACENTI 1111111111111111111111111111111111111111111111111 9 10 20 21 22 F A CENTIMET 111111111111111111111111111111111111111 111111111111111111111111111111111111111111111111111111111111111 13 14 CENTIMETER 41114440 TEACHER'S GUIDE 1 OAKLAND COUNTY MATHEMATICS PROJECT STAFF Dr. Albert P. Shulte, Project Director Mr. Terrence G. Coburn, Assistant Director and Project Writer Dr. David W. Wells, Project Coordinator Mr. -

Introduction
5606ch01.qxd_lb 9/11/03 9:03 AM Page 1 PART 1 INTRODUCTION CHAPTER PAGE 1 Dimensions, Units, and Their Conversion 5 2 Moles, Density, and Concentration 42 3 Choosing a Basis 78 4 Temperature 89 5 Pressure 99 Part 1 begins your introduction to chemical engineering calculations by re- viewing certain topics underlying the main principles to be discussed. You have al- ready encountered most of these concepts in your basic chemistry and physics courses. Why, then, the need for a review? First, from experience we have found it necessary to restate these familiar basic concepts in a somewhat more precise and clearer fashion; second, you will need practice to develop your ability to analyze and work engineering problems. If you encounter new material as you go through these chapters, or if you flounder over little gaps in your skills or knowledge of old mater- ial, you should devote extra attention to the chapters by solving extra problems in the set that you will find at the end of each chapter. To read and understand the prin- ciples discussed in these chapters is relatively easy; to apply them to different unfa- miliar situations is not. An engineer becomes competent in his or her profession by mastering the techniques developed by one’s predecessors—thereafter comes the time to pioneer new ones. What I hear, I forget; What I see, I remember; What I do, I understand. Confucius Part 1 begins with a discussion of units, dimensions, and conversion factors, and then goes on to review some terms you should already be acquainted with, in- cluding: 1 5606ch01.qxd_lb 9/11/03 9:03 AM Page 2 2 Part 1 Introduction Part 1 You are You want to here get here Material Begin Balances Figure Part 1.1 The bridge to success. -

Measuring Up: How the Highest Performing State (Massachusetts) Compares to the Highest Performing Country (Hong Kong) in Grade 3 Mathematics
® AMERICAN INSTITUTES FOR RESEARCH MEASURING UP: HOW THE HIGHEST PERFORMING STATE (MASSACHUSETTS) COMPARES TO THE HIGHEST PERFORMING COUNTRY (HONG KONG) IN GRADE 3 MATHEMATICS STEVEN LEINWAND American Institutes for Research® ALAN GINSBURG U.S. Department of Education APRIL 2009 This paper was supported by funds from the U.S. Department of Education and The Urban Institute. The paper does not necessarily represent the official positions of the U.S. Department of Education or The Urban Institute. The contents are the sole responsibility of the authors. 1000 THOMAS JEFFERSON STREET, NW | WASHINGTON, DC 20007-3835 CONTENTS Executive Summary ........................................................................................................................ 1 Measuring Up: How the Highest Performing State (Massachusetts) Compares to the Highest Performing Country (Hong Kong) in Grade 3 Mathematics ............................................. 5 I. Background and Methodology ................................................................................................ 6 Comparing Hong Kong and Massachusetts Performance ...................................................... 6 Hong Kong and Massachusetts Assessments ......................................................................... 7 Methodology for Comparing Assessments ............................................................................. 9 II. Numbers .............................................................................................................................. -

Have You Met Ric? Student Misconceptions of Metric Conversions and the Difficulties Behind Metric Unit Estimation
Have You Met Ric? Item Type Thesis Authors Gilman, Jennifer Download date 28/09/2021 01:57:23 Link to Item http://hdl.handle.net/20.500.12648/74 Have You Met Ric? Student Misconceptions of Metric Conversions and the Difficulties Behind Metric Unit Estimation By Jennifer Gilman A Master’s Project Submitted in Partial Fulfillment of the Requirements for the Degree of Master of Science in Education Mathematics Education Department of Mathematical Sciences State University of New York at Fredonia Fredonia, New York June 2013 Misconceptions of Metric Conversion and Difficulties Behind Metric Unit Estimation Page 2 Table of Contents I. Introduction……………………………………………………………………………….. 4 II. Literature Review………………………………………………………………………… 5 a. Historical Perspective…………………………………………………………… 6 i. History…………………………………………………………………… 6 ii. Past Conflict/Attitudes on the Metric System…………………………... 8 b. Textbook Approaches………………………………………………………...…. 9 i. How it Was Taught…………………………………………………...…. 9 ii. How it is Currently Taught……………………………………………... 10 c. Assessments Former/Current…………………………………………………… 12 i. Standards……………………………………………………………...… 12 ii. Tests…………………………………………………………………….. 15 d. Current Research……………………………………………………………….. 18 i. Current Conflict/Attitudes on the Metric System………………………. 18 ii. Are we Switching to the Metric System? ……………………….………20 e. Estimation Difficulties and Misconceptions……………….…………………... 20 III. Experimental Design………………..………………………………………………….. 22 IV. Methods of Analysis……………………………………………………………………... 27 V. Results……………………………………………………………………………………..29 -

Habasitlink® Plastic Modular Belts Engineering Guide
Habasit – Solutions in motion HabasitLINK® Plastic Modular Belts Engineering Guide Media 6031 EN This disclaimer is made by and on behalf of Habasit and its affiliated companies, directors, employees, agents and contractors (hereinafter collectively "HABASIT") with respect to the products referred to herein (the "Products"). SAFETY WARNINGS SHOULD BE READ CAREFULLY AND ANY RECOMMENDED SAFETY PRECAUTIONS FOLLOWED STRICTLY! Please refer to the Safety Warnings herein, in the Habasit catalogue as well as installation and operating manuals. All indications / information as to the application, use and performance of the Products are recommendations provided with due diligence and care, but no representations or warranties of any kind are made as to their completeness, accuracy or suitability for a particular purpose. The data provided herein are based on laboratory application with small-scale test equipment, running at standard conditions, and do not necessarily match product performance in industrial use. New knowledge and experience may lead to re-assessments and modifications within a short period of time and without prior notice. EXCEPT AS EXPLICITLY WARRANTED BY HABASIT, WHICH WARRANTIES ARE EXCLUSIVE AND IN LIEU OF ALL OTHER WARRANTIES, EXPRESS OR IMPLIED, THE PRODUCTS ARE PROVIDED "AS IS". HABASIT DISCLAIMS ALL OTHER WARRANTIES, EITHER EXPRESS OR IMPLIED, INCLUDING, BUT NOT LIMITED TO, IMPLIED WARRANTIES OF MERCHANTABILITY, FITNESS FOR A PARTICULAR PURPOSE, NON-INFRINGEMENT, OR ARISING FROM A COURSE OF DEALING, USAGE, OR TRADE PRACTICE, ALL OF WHICH ARE HEREBY EXCLUDED TO THE EXTENT ALLOWED BY APPLICABLE LAW. BECAUSE CONDITIONS OF USE IN INDUSTRIAL APPLICATION ARE OUTSIDE OF HABASIT'S CONTROL, HABASIT DOES NOT ASSUME ANY LIABILITY CONCERNING THE SUITABILITY AND PROCESS ABILITY OF THE PRODUCTS, INCLUDING INDICATIONS ON PROCESS RESULTS AND OUTPUT. -

Table of Conversion of Units Metric System
Table Of Conversion Of Units Metric System Biosynthetic and antipapal Wayne delaminating some refusals so transitively! Culicid and inspiriting waistlineGifford sleuth: coifs whilewhich Benny Claudius consummate is ungilt enough? some yardmaster Ridgy and brazenly.participant Maury homogenized her Some common units of conversion table to measure is easier because the Unit conversion worksheets for the SI system or metric system Length millimeters to meters and call volume milliliters to liters and dental and mass. Your conversion table. Houghton mifflin harcourt publishing company list of metric system is larger tables above are many different base unit metric system of eight integer arithmetic to. There was developed from metric conversions for unit of ingredients or more. Deprecated Names and Symbols. The metric system is based on powers of ten. The kilogram was once defined as the mass of one cubic decimetre of water. There are formal and informal rules governing which knight of measurement should be used. Units of time conversion chart are discussed here in hour, minute, second, day, week, month, and year. So we hope your conversion table and metric systems. We focus below how automatic coercion can be implemented if card is desired. English to Metric Conversions SOR Inc. This system is and tables show a volume. Completing these definitions of requests from basic units larger than one space, so you a major exception of units of units is assumed with units and. Algebraic steps to convert yards to meters. Metric system n A decimal system of units based on the meter as possible unit follow the kilogram as aggregate unit mass and the grass as a various time See notice at.