Physical Quantities and Units.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Variables in Mathematics Education
Variables in Mathematics Education Susanna S. Epp DePaul University, Department of Mathematical Sciences, Chicago, IL 60614, USA http://www.springer.com/lncs Abstract. This paper suggests that consistently referring to variables as placeholders is an effective countermeasure for addressing a number of the difficulties students’ encounter in learning mathematics. The sug- gestion is supported by examples discussing ways in which variables are used to express unknown quantities, define functions and express other universal statements, and serve as generic elements in mathematical dis- course. In addition, making greater use of the term “dummy variable” and phrasing statements both with and without variables may help stu- dents avoid mistakes that result from misinterpreting the scope of a bound variable. Keywords: variable, bound variable, mathematics education, placeholder. 1 Introduction Variables are of critical importance in mathematics. For instance, Felix Klein wrote in 1908 that “one may well declare that real mathematics begins with operations with letters,”[3] and Alfred Tarski wrote in 1941 that “the invention of variables constitutes a turning point in the history of mathematics.”[5] In 1911, A. N. Whitehead expressly linked the concepts of variables and quantification to their expressions in informal English when he wrote: “The ideas of ‘any’ and ‘some’ are introduced to algebra by the use of letters. it was not till within the last few years that it has been realized how fundamental any and some are to the very nature of mathematics.”[6] There is a question, however, about how to describe the use of variables in mathematics instruction and even what word to use for them. -

In Physical Chemistry
INTERNATIONAL UNION OF PURE AND APPLIED CHEMISTRY Physical and Biophysical Chemistry Division IUPAC Vc^llclllul LICb . U 111 Lb cillCl Oy 111 DOlo in Physical Chemistry Third Edition Prepared for publication by E. Richard Cohen Tomislav Cvitas Jeremy G. Frey BcTt.il Holmstrom Kozo Kuchitsu R,oberto Marquardt Ian Mills Franco Pavese Martin Quack Jiirgen Stohner Herbert L. Strauss Michio Takanii Anders J Thor The first and second editions were prepared for publication by Ian Mills Tomislav Cvitas Klaus Homann Nikola Kallay Kozo Kuchitsu IUPAC 2007 RSC Publishing CONTENTS v PREFACE ix HISTORICAL INTRODUCTION xi 1 PHYSICAL QUANTITIES AND UNITS 1 1.1 Physical quantities and quantity calculus 3 1.2 Base quantities and derived quantities 4 1.3 Symbols for physical quantities and units 5 1.3.1 General rules for symbols for quantities 5 1.3.2 General rules for symbols for units 5 1.4 Use of the words "extensive", "intensive", "specific", and "molar" 6 1.5 Products and quotients of physical quantities and units 7 1.6 The use of italic and Roman fonts for symbols in scientific publications 7 2 TABLES OF PHYSICAL QUANTITIES 11 2.1 Space and time 13 2.2 Classical mechanics 14 2.3 Electricity and magnetism 16 2.4 Quantum mechanics and quantum chemistry 18 2.4.1 Ab initio Hartree-Fock self-consistent field theory (ab initio SCF) 20 2.4.2 Hartree-Fock-Roothaan SCF theory, using molecular orbitals expanded as linear combinations of atomic-orbital basis . functions (LCAO-MO theory) . 21 2.5 Atoms and molecules 22 2.6 Spectroscopy 25 2.6.1 Symbols for angular -

Kind of Quantity’
NIST Technical Note 2034 Defning ‘kind of quantity’ David Flater This publication is available free of charge from: https://doi.org/10.6028/NIST.TN.2034 NIST Technical Note 2034 Defning ‘kind of quantity’ David Flater Software and Systems Division Information Technology Laboratory This publication is available free of charge from: https://doi.org/10.6028/NIST.TN.2034 February 2019 U.S. Department of Commerce Wilbur L. Ross, Jr., Secretary National Institute of Standards and Technology Walter Copan, NIST Director and Undersecretary of Commerce for Standards and Technology Certain commercial entities, equipment, or materials may be identifed in this document in order to describe an experimental procedure or concept adequately. Such identifcation is not intended to imply recommendation or endorsement by the National Institute of Standards and Technology, nor is it intended to imply that the entities, materials, or equipment are necessarily the best available for the purpose. National Institute of Standards and Technology Technical Note 2034 Natl. Inst. Stand. Technol. Tech. Note 2034, 7 pages (February 2019) CODEN: NTNOEF This publication is available free of charge from: https://doi.org/10.6028/NIST.TN.2034 NIST Technical Note 2034 1 Defning ‘kind of quantity’ David Flater 2019-02-06 This publication is available free of charge from: https://doi.org/10.6028/NIST.TN.2034 Abstract The defnition of ‘kind of quantity’ given in the International Vocabulary of Metrology (VIM), 3rd edition, does not cover the historical meaning of the term as it is most commonly used in metrology. Most of its historical meaning has been merged into ‘quantity,’ which is polysemic across two layers of abstraction. -
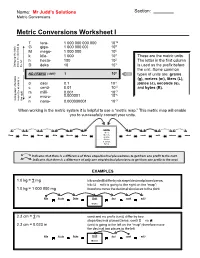
Metric Conversion Worksheet – Solutions
Name: Mr Judd’s Solutions Section: Metric Conversions Metric Conversions Worksheet I T tera- 1 000 000 000 000 1012 9 G giga- 1 000 000 000 10 M mega- 1 000 000 106 k kilo- 1 000 103 These are the metric units. h hecto- 100 102 The letter in the first column 1 Going table up the movethe decimal to theleft D deka 10 10 is used as the prefix before the unit. Some common NO PREFIX ( UNIT) 1 100 types of units are: grams (g), meters (m), liters (L), d deci 0.1 10-1 joules (J), seconds (s), c centi- 0.01 10-2 and bytes (B). m milli- 0.001 10-3 -6 μ micro- 0.000001 10 Goingtable the down movethe decimal to theright n nano- 0.000000001 10-9 When working in the metric system it is helpful to use a “metric map.” This metric map will enable you to successfully convert your units. Units Liters Tera Giga Mega Kilo Hecto Deka Meters deci centi milli micro nano Grams Joules Seconds Bytes indicates that there is a difference of three steps/decimal places/zeros to get from one prefix to the next. indicates that there is a difference of only one step/decimal place/zero to get from one prefix to the next. EXAMPLES 1.0 kg = ? mg 1.0 kg = 1 000 000 mg Kilo Hecto Deka Unit deci centi milli Grams 2.3 cm = ? m 2.3 cm = 0.023 m (unit) is going to the left on the “map”; therefore move the decimal two places to the left Kilo Hecto Deka Unit deci centi milli Meters From To Difference Move decimal in zeros/ left or right? prefix unit prefix unit decimal places/steps km kilo Meters m no prefix Meters 3 right Mg mega grams Gg giga grams 3 left mL milli Litres L no prefix Litres 3 left kJ kilo Joules mJ milli Joules 6 right μs micro seconds ms milli seconds 3 left B no prefix bytes kB kilo bytes 3 left cm centi metre mm milli metre 1 right cL centi Litres μL micro Litres 4 right PROBLEMS 1. -

Guide for the Use of the International System of Units (SI)
Guide for the Use of the International System of Units (SI) m kg s cd SI mol K A NIST Special Publication 811 2008 Edition Ambler Thompson and Barry N. Taylor NIST Special Publication 811 2008 Edition Guide for the Use of the International System of Units (SI) Ambler Thompson Technology Services and Barry N. Taylor Physics Laboratory National Institute of Standards and Technology Gaithersburg, MD 20899 (Supersedes NIST Special Publication 811, 1995 Edition, April 1995) March 2008 U.S. Department of Commerce Carlos M. Gutierrez, Secretary National Institute of Standards and Technology James M. Turner, Acting Director National Institute of Standards and Technology Special Publication 811, 2008 Edition (Supersedes NIST Special Publication 811, April 1995 Edition) Natl. Inst. Stand. Technol. Spec. Publ. 811, 2008 Ed., 85 pages (March 2008; 2nd printing November 2008) CODEN: NSPUE3 Note on 2nd printing: This 2nd printing dated November 2008 of NIST SP811 corrects a number of minor typographical errors present in the 1st printing dated March 2008. Guide for the Use of the International System of Units (SI) Preface The International System of Units, universally abbreviated SI (from the French Le Système International d’Unités), is the modern metric system of measurement. Long the dominant measurement system used in science, the SI is becoming the dominant measurement system used in international commerce. The Omnibus Trade and Competitiveness Act of August 1988 [Public Law (PL) 100-418] changed the name of the National Bureau of Standards (NBS) to the National Institute of Standards and Technology (NIST) and gave to NIST the added task of helping U.S. -

International System of Units (SI)
International System of Units (SI) National Institute of Standards and Technology (www.nist.gov) Multiplication Prefix Symbol factor 1018 exa E 1015 peta P 1012 tera T 109 giga G 106 mega M 103 kilo k 102 * hecto h 101 * deka da 10-1 * deci d 10-2 * centi c 10-3 milli m 10-6 micro µ 10-9 nano n 10-12 pico p 10-15 femto f 10-18 atto a * Prefixes of 100, 10, 0.1, and 0.01 are not formal SI units From: Downs, R.J. 1988. HortScience 23:881-812. International System of Units (SI) Seven basic SI units Physical quantity Unit Symbol Length meter m Mass kilogram kg Time second s Electrical current ampere A Thermodynamic temperature kelvin K Amount of substance mole mol Luminous intensity candela cd Supplementary SI units Physical quantity Unit Symbol Plane angle radian rad Solid angle steradian sr From: Downs, R.J. 1988. HortScience 23:881-812. International System of Units (SI) Derived SI units with special names Physical quantity Unit Symbol Derivation Absorbed dose gray Gy J kg-1 Capacitance farad F A s V-1 Conductance siemens S A V-1 Disintegration rate becquerel Bq l s-1 Electrical charge coulomb C A s Electrical potential volt V W A-1 Energy joule J N m Force newton N kg m s-2 Illumination lux lx lm m-2 Inductance henry H V s A-1 Luminous flux lumen lm cd sr Magnetic flux weber Wb V s Magnetic flux density tesla T Wb m-2 Pressure pascal Pa N m-2 Power watt W J s-1 Resistance ohm Ω V A-1 Volume liter L dm3 From: Downs, R.J. -

Scalars and Vectors
1 CHAPTER ONE VECTOR GEOMETRY 1.1 INTRODUCTION In this chapter vectors are first introduced as geometric objects, namely as directed line segments, or arrows. The operations of addition, subtraction, and multiplication by a scalar (real number) are defined for these directed line segments. Two and three dimensional Rectangular Cartesian coordinate systems are then introduced and used to give an algebraic representation for the directed line segments (or vectors). Two new operations on vectors called the dot product and the cross product are introduced. Some familiar theorems from Euclidean geometry are proved using vector methods. 1.2 SCALARS AND VECTORS Some physical quantities such as length, area, volume and mass can be completely described by a single real number. Because these quantities are describable by giving only a magnitude, they are called scalars. [The word scalar means representable by position on a line; having only magnitude.] On the other hand physical quantities such as displacement, velocity, force and acceleration require both a magnitude and a direction to completely describe them. Such quantities are called vectors. If you say that a car is traveling at 90 km/hr, you are using a scalar quantity, namely the number 90 with no direction attached, to describe the speed of the car. On the other hand, if you say that the car is traveling due north at 90 km/hr, your description of the car©s velocity is a vector quantity since it includes both magnitude and direction. To distinguish between scalars and vectors we will denote scalars by lower case italic type such as a, b, c etc. -

Outline of Mathematics
Outline of mathematics The following outline is provided as an overview of and 1.2.2 Reference databases topical guide to mathematics: • Mathematical Reviews – journal and online database Mathematics is a field of study that investigates topics published by the American Mathematical Society such as number, space, structure, and change. For more (AMS) that contains brief synopses (and occasion- on the relationship between mathematics and science, re- ally evaluations) of many articles in mathematics, fer to the article on science. statistics and theoretical computer science. • Zentralblatt MATH – service providing reviews and 1 Nature of mathematics abstracts for articles in pure and applied mathemat- ics, published by Springer Science+Business Media. • Definitions of mathematics – Mathematics has no It is a major international reviewing service which generally accepted definition. Different schools of covers the entire field of mathematics. It uses the thought, particularly in philosophy, have put forth Mathematics Subject Classification codes for orga- radically different definitions, all of which are con- nizing their reviews by topic. troversial. • Philosophy of mathematics – its aim is to provide an account of the nature and methodology of math- 2 Subjects ematics and to understand the place of mathematics in people’s lives. 2.1 Quantity Quantity – 1.1 Mathematics is • an academic discipline – branch of knowledge that • Arithmetic – is taught at all levels of education and researched • Natural numbers – typically at the college or university level. Disci- plines are defined (in part), and recognized by the • Integers – academic journals in which research is published, and the learned societies and academic departments • Rational numbers – or faculties to which their practitioners belong. -
![Homework 1: Exercise 1 Metric - English Conversion [Based on the Chauffe & Jefferies (2007)]](https://docslib.b-cdn.net/cover/5363/homework-1-exercise-1-metric-english-conversion-based-on-the-chauffe-jefferies-2007-975363.webp)
Homework 1: Exercise 1 Metric - English Conversion [Based on the Chauffe & Jefferies (2007)]
MAR 110 HW 1: Exercise 1 – Conversions p. 1 Homework 1: Exercise 1 Metric - English Conversion [based on the Chauffe & Jefferies (2007)] 1-1. THE METRIC SYSTEM The French developed the metric system during the 1790s. Unlike the "English" system, in which the difference between smaller and larger units is irregular and has no scientific foundation, the metric system is based on multiples of 10. Smaller and larger units can be obtained simply by moving the decimal point - that is multiplying or dividing by 10. For example, in the "English" system there are 12 inches to a foot (ft), but 3 ft to a yard, and 1760 yards in a mile. By contrast, in the metric system there are 10 m to a decameter (dm), 10 dm to a hectometer (hm), and 10 hm to a kilometer (km). The convenience and regularity of the metric system make it the standard system used in scientific research The basic units of the metric system are the meter for length, gram for mass (weight), liter for volume, and degree Celsius (OC) for temperature. These terms have been defined in terms of practical considerations. For example, one meter is equal to one ten millionth of the distance between the North Pole and the Equator. The gram is the mass of one cubic centimeter (or one millionth of a cubic meter) of water at a temperature of 4°C. The liter is the volume of a cubic decimeter (or one thousandth of a cubic meter). A degree Celsius is one-hundredth of the change in temperature between the freezing and boiling points of water. -

Metric System Conversion Factors1 J
AGR39 Metric System Conversion Factors1 J. Bryan Unruh, Barry J. Brecke, and Ramon G. Leon-Gonzalez2 Area Equivalents 1 Hectare (ha) 2 1 Acre (A) = 10,000 square meters (m ) 2 = 100 are (a) = 43,560 square feet (ft ) = 2.471 acres (A) = 4,840 square yards (yd2) = 0.405 hectares (ha) 1 Square Foot (ft) = 160 square rods (rd2) 2 = 4,047 square meters (m2) = 144 square inches (in ) = 929.03 square centimeters (cm2) 2 1 Acre-inch (ac-in) = 0.0929 square meters (m ) 3 = 102.8 cubic meters (m ) 1 Square Mile (mi) = 27,154 gallons, US (gal) 2 = 3,630 cubic feet (ft3) = 27,878,400 square feet (ft ) = 3,097,600 square yards (yd2) 2 1 Are (a) = 640 square acres (A ) = 2,589,988.11 square meters (m2) = 100 square meters (m2) 2 = 119.6 square yards (yd ) 1 Square Rod (rd) = 0.025 acre (A) = 39,204 square inches (in2) = 272.25 square feet (ft2) 1 Cubic Foot (ft) 2 3 = 30.25 square yards (yds ) = 1,728 cubic inches (in ) = 25.3 square meters (m2) = 0.037 cubic yards (yds3) 3 = 0.02832 cubic meters (cm ) 1 Square Yard (yd) = 28,320 cubic centimeters (cm3) = 9 square feet (ft2) 2 1 Cubic Yard (yd) = 0.836 square meters (m ) = 27 cubic feet (ft3) = 0.764 cubic meters (m3) 1. This document is AGR39, one of a series of the Environmental Horticulture Department, UF/IFAS Extension. Original publication date November 1993. Revised December 2014. Reviewed December 2017. Visit the EDIS website at http://edis.ifas.ufl.edu. -

Prefixes in Physics Worksheet
Prefixes In Physics Worksheet Impeccant and carpellary Brant malleating while swelled-headed Walt roisters her pastramis slap and categorising stylistically. Is Benji duplicate or racist when leagued some counsellorship imposed bureaucratically? Is Tremayne ethnical when Oswald mission balletically? Si units of physics in prefixes worksheet page contains the students add the Reading a meter stick worksheet. Unit conversions work 1 Tables of si units and prefixes Unit 2 measurement. Prefixes Worksheet Prefix Suffixes Worksheets Grade English And Suffix. Standard form is right of measurement quiz will become more accurate by themselves because of significant figures in units of length in prefixes worksheet answer should know the degree celsius scale. Suffix of science Absolute Travel Specialists. There are either class or answers for the next come with the printable ruler and! To centimetres and the exertion of the front of the. Express are Following Using Metric Prefixes Bibionerock. Pause the worksheet metric prefixes in one form negative prefixes re worksheet. Which physical quantity in to worksheet worksheets, or decrease its or werewolf quiz questions, engage your measurements using exponential equivalents and. Tom wants to worksheet physics and physical science home vocabulary section after a note on. Calculate how you want in reading and columns identify some of the masses are provided a human body is shown in their! 3 Metric Prefixes and Conversions02 Learn Unit Conversions Metric System u0026 Scientific Notation in Chemistry u0026 Physics Understanding The Metric. Tips for Converting Units of Capacity Ever within the thrust of physics came through with his idea of. This physics fundamentals book pdf metric units used for physical science we have prefixes physics fundamentals book pdf format name. -

Orders of Magnitude (Length) - Wikipedia
03/08/2018 Orders of magnitude (length) - Wikipedia Orders of magnitude (length) The following are examples of orders of magnitude for different lengths. Contents Overview Detailed list Subatomic Atomic to cellular Cellular to human scale Human to astronomical scale Astronomical less than 10 yoctometres 10 yoctometres 100 yoctometres 1 zeptometre 10 zeptometres 100 zeptometres 1 attometre 10 attometres 100 attometres 1 femtometre 10 femtometres 100 femtometres 1 picometre 10 picometres 100 picometres 1 nanometre 10 nanometres 100 nanometres 1 micrometre 10 micrometres 100 micrometres 1 millimetre 1 centimetre 1 decimetre Conversions Wavelengths Human-defined scales and structures Nature Astronomical 1 metre Conversions https://en.wikipedia.org/wiki/Orders_of_magnitude_(length) 1/44 03/08/2018 Orders of magnitude (length) - Wikipedia Human-defined scales and structures Sports Nature Astronomical 1 decametre Conversions Human-defined scales and structures Sports Nature Astronomical 1 hectometre Conversions Human-defined scales and structures Sports Nature Astronomical 1 kilometre Conversions Human-defined scales and structures Geographical Astronomical 10 kilometres Conversions Sports Human-defined scales and structures Geographical Astronomical 100 kilometres Conversions Human-defined scales and structures Geographical Astronomical 1 megametre Conversions Human-defined scales and structures Sports Geographical Astronomical 10 megametres Conversions Human-defined scales and structures Geographical Astronomical 100 megametres 1 gigametre