Full Article
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

University of Cape Town
The effects of introduced mice on seabirds breeding at sub-Antarctic Islands Ben J. Dilley Thesis presented for the degree of Doctor of Philosophy Town FitzPatrick Institute of African Ornithology DST/NRF Centre of Excellence Department of Biological Sciences, Faculty of Science University of CapeCape Town of June 2018 University Supervised by Professor Peter G. Ryan The copyright of this thesis vests in the author. No quotation from it or information derivedTown from it is to be published without full acknowledgement of the source. The thesis is to be used for private study or non- commercial research purposes Capeonly. of Published by the University of Cape Town (UCT) in terms of the non-exclusive license granted to UCT by the author. University Declaration This thesis reports original research that I conducted under the auspices of the FitzPatrick Institute, University of Cape Town. All assistance received has been fully acknowledged. This work has not been submitted in any form for a degree at another university. ………………….................. Ben J. Dilley Cape Town, June 2018 i A 10 day-old great shearwater Ardenna gravis chick being attacked by an invasive House mouse Mus musculus in an underground burrow on Gough Island in 2014 (photo Ben Dilley). ii Table of Contents Page Abstract ....................................................................................................................................... iv Acknowledgements .......................................................................................................................... vi Chapter 1 General introduction: Islands, mice and seabirds ......................................................... 1 Chapter 2 Clustered or dispersed: testing the effect of sampling strategy to census burrow-nesting petrels with varied distributions at sub-Antarctic Marion Island ...... 13 Chapter 3 Modest increases in densities of burrow-nesting petrels following the removal of cats Felis catus from sub-Antarctic Marion Island ................................... -

Grey Petrels Returning to Campbell Island? Survey and Census 14 Years After Rodent Eradication
Parker et al. 2015 Are grey petrels returning to Campbell Island? Survey and census 14 years after rodent eradication Graham C. Parker, Kalinka Rexer-Huber, David Thompson Report to the Department of Conservation June 2015 __________________________________________________________________________________ Grey petrels, Campbell Island 1 Parker et al. 2015 Are grey petrels returning to Campbell Island? Survey and census 14 years after rodent eradication Report to the Department of Conservation Graham C. Parker 1*, Kalinka Rexer-Huber 1, 3, David Thompson 2 1 Parker Conservation, 126 Maryhill Terrace, Dunedin New Zealand 2 National Institute for Water and Atmosphere (NIWA), 301 Evans Bay Parade, Hataitai, Wellington New Zealand 3 Department of Zoology, University of Otago, 340 Great King Street, Dunedin New Zealand * Author for correspondence: [email protected] Please cite as: Parker, G.C., Rexer-Huber, K. and Thompson, D. 2015. Are grey petrels returning to Campbell Island? Survey and census 14 years after rodent eradication. Unpublished report to the Department of Conservation. Parker Conservation, Dunedin __________________________________________________________________________________ Grey petrels, Campbell Island 2 Parker et al. 2015 Contents Abstract ...................................................................................................................................... 3 Introduction ............................................................................................................................... -

Procellaria Cinerea) on Antipodes Island, New Zealand
269 Notornis, 2013, Vol. 60: 269-278 0029-4470 © The Ornithological Society of New Zealand, Inc. Notes on the distribution, behaviour and status of grey petrel (Procellaria cinerea) on Antipodes Island, New Zealand ELIZABETH. A. BELL* PO Box 607, Blenheim, Marlborough 7240, New Zealand BRIAN D. BELL 35 Selmes Road, Rapaura, RD3, Blenheim 7273, New Zealand J. L. SIM 178 C.D. Farm Road, Ohau, New Zealand M.J. IMBER** 6 Hillcrest Lane, Levin 5500, New Zealand Abstract Aspects of the breeding biology of the grey petrel (Procellaria cinerea) were studied on Antipodes Island between April and June 2001. The island was surveyed to determine grey petrel distribution and four 2500 m2 census grids were established. The survey suggested that the distribution of grey petrels was restricted to steep, well-draining areas dominated by Poa litorosa tussock (approximately 510 ha of the 2025 ha island). Occupied burrow density within the 4 census grids ranged from 31 to 44 burrows (0.01 burrows per square metre). Extrapolating from the census grid density to the total grey petrel habitat resulted in a population estimate of 114,730 birds: 53,000 breeding pairs (range = 32,000- 73,000) and 8,670 non-breeding-birds (range = 4,000-16,320) were present on Antipodes Island. Aspects of the behaviour of the species were recorded. Comparisons are made with other members of the genus Procellaria. Bell, E.A.; Bell, B.D.; Sim, J.L.; Imber, M.J. 2013. Notes on the distribution, behaviour and status of grey petrel (Procellaria cinerea) on Antipodes Island, New Zealand. -
Grey Petrel Procellaria Cinerea (Entire Population) in Appendix II of the Convention on the Conservation of Migratory Species of Wild Animals
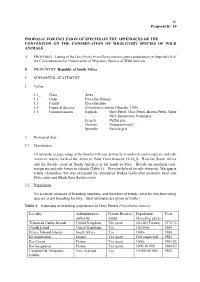
81 Proposal II / 10 PROPOSAL FOR INCLUSION OF SPECIES ON THE APPENDICES OF THE CONVENTION ON THE CONSERVATION OF MIGRATORY SPECIES OF WILD ANIMALS A. PROPOSAL: Listing of the Grey Petrel Procellaria cinerea (entire population) in Appendix II of the Convention on the Conservation of Migratory Species of Wild Animals. B. PROPONENT: Republic of South Africa. C. SUPPORTING STATEMENT 1. Taxon 1.1_ Class Aves 1.2 Order Procellariiformes 1.3 Family Procellariidae 1.4 Genus & Species Procellaria cinerea (Gmelin, 1789) 1.5 Common names English: Grey Petrel, Gray Petrel, Brown Petrel, Great Grey Shearwater, Pediunker French: Puffin gris German: Grausturmvogel Spanish: Pardela gris 2. Biological data 2.1 Distribution Circumpolar pelagic range in the Southern Ocean, primarily in southern cool-temperate and sub- Antarctic waters north of the Antarctic Polar Front between 32-58S. Reaches South Africa and the Pacific coast of South America as far north as Peru. Breeds on southern cool- temperate and sub-Antarctic islands (Table 1). Previously bred on sub-Antarctic Macquarie Island (Australia), but was extirpated by introduced Wekas Gallirallus australis, feral cats Felis catus and Black Rats Rattus rattus. 2.2 Population No accurate censuses of breeding numbers, and therefore of trends, exist for this burrowing species at any breeding locality. Best estimates are given in Table 1. Table 1. Estimates of breeding populations of Grey Petrels Procellaria cinerea Locality Administrative Nature Reserve Population Year authority status (breeding pairs) Tristan da Cunha Islands United Kingdom Yes (part) 50-100 (Tristan) 1972/74 Gough Island United Kingdom Yes 100 000s 1984 Prince Edward Islands South Africa Yes 1000s 1984 Ile Amsterdam France Yes (part) Few suspected 1983 Iles Crozet France Yes (part) 1000s 1981/82 Iles Kerguelen France Yes (part) 5000-10 000 1984-87 Campbell & Antipodes New Zealand Yes 10 000-50 000 1984 Islands 82 Proposal II / 10 2.3 Habitat Marine, in southern coastal and pelagic waters. -

Action Plan for Seabird Conservation in New Zealand Part B: Non-Threatened Seabirds
Action Plan for Seabird Conservation in New Zealand Part B: Non-Threatened Seabirds THREATENED SPECIES OCCASIONAL PUBLICATION NO. 17 Action Plan for Seabird Conservation in New Zealand Part B: Non-Threatened Seabirds THREATENED SPECIES OCCASIONAL PUBLICATION NO. 17 by Graeme A. Taylor Published by Biodiversity Recovery Unit Department of Conservation PO Box 10-420 Wellington New Zealand Illustrations Front cover: Northern diving petrel, North Brothers Island, 1998 Inside front cover: Brown skua, Campbell Island, 1986 Source of illustrations All photographs were taken by the author unless stated otherwise. © May 2000, Department of Conservation ISSN 1170-3709 ISBN 0-478-21925-3 Cataloguing in Publication Taylor, Graeme A. Action plan for seabird conservation in New Zealand. Part B, Non-threatened seabirds / by Graeme A. Taylor. Wellington, N.Z. : Dept. of Conservation, Biodiversity Recovery Unit, 2000. 1. v. ; 30 cm. (Threatened Species occasional publication, 1170-3709 ; 17.) Cataloguing-in-Publication data. - Includes bibliographical references. ISBN 0478219253 1. Sea birds— New Zealand. 2. Rare birds—New Zealand. I. New Zealand. Biodiversity Recovery Unit. II. Title. Series: Threatened species occasional publication ; 17. 236 CONTENTS PART A: THREATENED SEABIRDS Abbreviations used in Parts A and B 7 Abstract 9 1 Purpose 11 2 Scope and limitations 12 3 Sources of information 12 4 General introduction to seabirds 13 4.1 Characteristics of seabirds 14 4.2 Ecology of seabirds 14 4.3 Life history traits of seabirds 15 5 New Zealand seabirds -

Threats to Seabirds: a Global Assessment 2 3 4 Authors: Maria P
1 Threats to seabirds: a global assessment 2 3 4 Authors: Maria P. Dias1*, Rob Martin1, Elizabeth J. Pearmain1, Ian J. Burfield1, Cleo Small2, Richard A. 5 Phillips3, Oliver Yates4, Ben Lascelles1, Pablo Garcia Borboroglu5, John P. Croxall1 6 7 8 Affiliations: 9 1 - BirdLife International. The David Attenborough Building, Pembroke Street Cambridge CB2 3QZ UK 10 2 - BirdLife International Marine Programme, RSPB, The Lodge, Sandy, SG19 2DL 11 3 – British Antarctic Survey. Natural Environment Research Council, High Cross, Madingley Road, 12 Cambridge CB3 0ET, UK 13 4 – Centre for the Environment, Fishery and Aquaculture Science, Pakefield Road, Lowestoft, NR33, UK 14 5 - Global Penguin Society, University of Washington and CONICET Argentina. Puerto Madryn U9120, 15 Chubut, Argentina 16 * Corresponding author: Maria Dias, [email protected]. BirdLife International. The David 17 Attenborough Building, Pembroke Street Cambridge CB2 3QZ UK. Phone: +44 (0)1223 747540 18 19 20 Acknowledgements 21 We are very grateful to Bartek Arendarczyk, Sophie Bennett, Ricky Hibble, Eleanor Miller and Amy 22 Palmer-Newton for assisting with the bibliographic review. We thank Rachael Alderman, Pep Arcos, 23 Jonathon Barrington, Igor Debski, Peter Hodum, Gustavo Jimenez, Jeff Mangel, Ken Morgan, Paul Sagar, 24 Peter Ryan, and other members of the ACAP PaCSWG, and the members of IUCN SSC Penguin Specialist 25 Group (Alejandro Simeone, Andre Chiaradia, Barbara Wienecke, Charles-André Bost, Lauren Waller, Phil 26 Trathan, Philip Seddon, Susie Ellis, Tom Schneider and Dee Boersma) for reviewing threats to selected 27 species. We thank also Andy Symes, Rocio Moreno, Stuart Butchart, Paul Donald, Rory Crawford, 28 Tammy Davies, Ana Carneiro and Tris Allinson for fruitful discussions and helpful comments on earlier 29 versions of the manuscript. -

SEABIRD BYCATCH IDENTIFICATION GUIDE UPDATED AUGUST 2015 2 How to Use This Guide
SEABIRD BYCATCH IDENTIFICATION GUIDE UPDATED AUGUST 2015 2 How to use this guide 1. Identify the bird • Start by looking at its bill - size and position of nostrils as shown on pages 6-9 to decide if it’s an albatross, a petrel or another group. • If it’s an albatross, use the keys and photos on pages 10-13, to identify the bird to a particular species (or to the 2 or 3 species that it might be), and go to the page specified to confirm the identification. If it’s a petrel, use the key on pages 14-15 , then go to the page as directed. If it’s a shearwater, look at pages 66-77. 2. Record Record your identification in the logbook choosing one of the FAO codes, or a combination of codes from the list on pages 96-99. 3. Take photos Take three photos of the bird as shown on pages 78-81 and submit with the logbook. 4. Sample feathers If a sampling programme is in place, pluck some feathers for DNA analysis as shown on pages 82-83. SEABIRD BYCATCH IDENTIFICATION GUIDE 3 Contents How to use this guide 2 Measuring bill and wing length 4 Albatross, Petrel or other seabird? 6 Bill guide 8 Albatross key 10 Diomedea albatross key 12 Juvenile/Immature Thalassarche key 13 Petrel key 14 North Pacific Albatrosses 16 - 21 Waved Albatross 22 Phoebetria albatrosses (light-mantled and sooty) 24 - 27 Royal albatrosses 28 - 29 ‘Wandering-type’ albatrosses 30 - 37 Thalassarche albatrosses 38 - 51 Juvenile/Immature Thalassarche albatrosses 52 - 53 Giant petrels 54 - 55 Procellaria petrels 56 - 61 Other Petrels 62 - 65 Shearwaters 66 - 77 Data collection protocols - taking photos 78 Data collection protocols - examples of photos 80 Data collection protocols - feather samples for DNA analysis 82 Leg Bands 84 References 88 Your feedback 91 Hook Removal from Seabirds 92 Albatross species list 96 Petrel and Shearwater species list 98 4 Measuring Bill & Wing Length BILL LENGTH WING LENGTH 10 20 Ruler 30 (mm) 40 50 60 70 80 90 100 110 120 130 140 150 160 170 180 190 200 6 Albatross, Petrel, Shearwater Albatrosses Page 10 Separate nostrils. -

Detecting Cryptic Burrowing Petrels Recovery Post Eradication in a Remote Landscape
Detecting cryptic burrowing petrels recovery post eradication in a remote landscape By Julie McInnes In collaboration with: Jez Bird, Bruce Deagle, Rachael Alderman and Justine Shaw National Environmental Science Programme 4.2.3.4 August 2019 Cite this publication as: McInnes, J. with Bird, J., Deagle, B., Alderman, R, Shaw, J. 2019. Detecting cryptic burrowing petrels recovery post eradication in a remote landscape. NESP Threatened Species Recovery Hub Project 4.2.3.4 report, Brisbane. Cover image: White-headed petrel. This is one of several burrowing petrel species that are recovering on Macquarie Island following invasive species eradication. Image:Jez Bird. 2 Contents Executive Summary ......................................................................................................................................................................................... 4 Introduction ....................................................................................................................................................................................................... 4 Methods .............................................................................................................................................................................................................. 5 Sample collection ................................................................................................................................................................................... 5 Primer design .......................................................................................................................................................................................... -

MO 42 2 137-141.Pdf
Rexer-Huber et al.: Burrow occupancy and population of Atlantic Petrel 137 BURROW OCCUPANCY AND POPULATION SIZE IN THE ATLANTIC PETREL PTERODROMA INCERTA: A COMPARISON OF METHODS KALINKA REXER-HUBER1,2, GRAHAM C. PARKER1,2, PETER G. RYAN2 & RICHARD J. CUTHBERT1 1Royal Society for the Protection of Birds, Sandy, Bedfordshire SG19 2DL, England ([email protected]) 2Percy FitzPatrick Institute, DST/NRF Centre of Excellence, University of Cape Town, Rondebosch 7701, South Africa Received 14 July 2013, accepted 18 November 2013 SUMMARY REXER-HUBER, K., PARKER, G.C., RYAN, P.G. & CUTHBERT, R.J. 2014. Burrow occupancy and population size in the Atlantic Petrel Pterodroma incerta: a comparison of methods. Marine Ornithology 42: 137–141. To test the accuracy of burrowing seabird monitoring techniques, data from activity signs and playback methods were compared with burrowscope data from 652 Atlantic Petrel Pterodroma incerta burrows on Gough Island in the South Atlantic Ocean. In addition, burrow density was monitored over seven breeding seasons (2001–2012), burrow occupancy was assessed, and the Atlantic Petrel population estimate refined using the most recent density and occupancy figures. Activity signs and call playback have limited utility for monitoring this species. Activity signs overestimated actual burrow occupancy by 38% and playback underestimated occupancy by 76%. Playback utility may also be affected by density-related response priming. Mean burrow density using direct surveys was 0.19 ± 0.02 burrows/m2 (mean ± SE) over seven breeding seasons. There was no significant trend in burrow density 2001–2012, but burrow density varied significantly among years. Burrow occupancy was 52% (95% CI 48%–56%) and 31% (27%–36%) in 2010 and 2012, respectively. -

SEABIRD IDENTIFICATION CARDS for Fishing Vessels Operating in the Indian Ocean
SEABIRD IDENTIFICATION CARDS for Fishing Vessels operating in the Indian Ocean Indian Ocean Tuna Commission Commission des Thons de l’Océan Indien These seabird identification cards are produced as part of a series of awareness materials developed by the Indian Ocean Tuna Commission in order to improve the reporting of interactions between vessels targeting species under the management mandate of the IOTC and seabirds. This publication was made possible through financial assistance provided by IOTC. For further information, contact: Indian Ocean Tuna Commission Le Chantier Mall PO Box 1011, Victoria, SEYCHELLES Phone: +248.422.54.94 Fax: +248.422.43.64 Email: [email protected] Website: http://www.iotc.org Acknowledgements: We gratefully acknowledge contributions from Birdlife International and the Secretariat of ACAP for the development of these seabird identification cards. Illustrations by Peter Hayman, reproduced with permission of Random House Struik Publishers from Sasol Birds of Southern Africa. Photos courtesy of Dr. Ross Wanless, Projeto Albatroz/Fabiano Peppes, Albatross Task Force/BirdLife South Africa. ©Copyright: IOTC, 2011. Design and layout: Julien Million. Seabirds are species that derive their sustenance primarily from the ocean and which spend the bulk of their time (when not on land at breeding sites) at sea. Seabirds are characterised as being late to mature and slow to reproduce; some do not start to breed until they are ten years old. To compensate for this, seabirds are long-lived, with natural adult mortality typically very low. These traits make any increase in human-induced adult mortality potentially damaging for population viability, as even small increases in mortality can result in population declines. -

The Response of Burrow-Nesting Petrels and Other Vulnerable Bird Species to Vertebrate Pest Management and Climate Change on Sub-Antarctic Macquarie Island
Papers and Proceedings ofthe Royal Society of Tasmania, Volume 142(1), 2008 123 THE RESPONSE OF BURROW-NESTING PETRELS AND OTHER VULNERABLE BIRD SPECIES TO VERTEBRATE PEST MANAGEMENT AND CLIMATE CHANGE ON SUB-ANTARCTIC MACQUARIE ISLAND by Nigel Brothers & Catherine Bone (with nine text-figures and 17 tables) Brothers, N. &Bone, C. 2008 (31 :x) Theresponse ofburrow-nesting petrels and other vulnerable bird species to vertebrate pest management and climate change on sub-Antarctic Macquarie Island. Papers and Proceedings of the Royal Society of Tasmania I 42(1): 123-148. https://doi.org/10.26749/rstpp.142.1.123 ISSN 0080-4703. Department of Primary Industries, Water and Environment, GPO Box 44, Hobart, Tasmania 7001, Australia (NB), 178 South Arm Drive, Wonga Beach, Queensland 4873, Australia (NB*, CB) *Author and address for correspondence. Email: [email protected] Pest species management is causing rapid and significant changes to burrow-nesting petrel populations on sub-Antarctic Macquarie Island. The Weka, Gallirallus australis, was eliminated by 1989 and the Feral Cat, Felis catus, eradicated in 2000. The most abundant burrow nesting petrel species currently, White-headed Petrels, Pterodroma lessonii, Antarctic Prions, Pachypti!.a desolata, and Sooty Shearwaters, Puffinus griseus, have yet to increase in numbers, but are expected to do so in the absence of cats. This study found evidence that Grey Petrels, Procellaria cinerea, began breeding again on the island in 1999, after an absence of over 100 years. Blue Petrels, Halobaena caerulea, and Fairy Prions, Pachyptila turtur, were found to be re-colonising Macquarie Island from offshore stacks after a similar absence. -

Seabird Conservation Status, Threats and Priority Actions: a Global Assessment
Bird Conservation International (2012) 22:1–34. © BirdLife International, 2012 doi:10.1017/S0959270912000020 Seabird conservation status, threats and priority actions: a global assessment JOHN P. CROXALL, STUART H. M. BUTCHART, BEN LASCELLES, ALISON J. STATTERSFIELD, BEN SULLIVAN, ANDY SYMES and PHIL TAYLOR Summary We review the conservation status of, and threats to, all 346 species of seabirds, based on BirdLife International’s data and assessments for the 2010 IUCN Red List. We show that overall, seabirds are more threatened than other comparable groups of birds and that their status has deteriorated faster over recent decades. The principal current threats at sea are posed by commercial fisheries (through competition and mortality on fishing gear) and pollution, whereas on land, alien invasive predators, habitat degradation and human disturbance are the main threats. Direct exploitation remains a problem for some species both at sea and ashore. The priority actions needed involve: a) formal and effective site protection, especially for Important Bird Area (IBA) breeding sites and for marine IBA feeding and aggregation sites, as part of national, regional and global networks of Marine Protected Areas; b) removal of invasive, especially predatory, alien species (a list of priority sites is provided), as part of habitat and species recovery initiatives; and c) reduction of bycatch to negligible levels, as part of comprehensive implementation of ecosystem approaches to fisheries. The main knowledge gaps and research priorities relate to the three topics above but new work is needed on impacts of aquaculture, energy generation operations and climate change (especially effects on the distribution of prey species and rise in sea level).