PAEWG-02-08 Albatross and Petrel Distribution Within the South Indian
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Conservation Problems on Tristan Da Cunha Byj
28 Oryx Conservation Problems on Tristan da Cunha ByJ. H. Flint The author spent two years, 1963-65, as schoolmaster on Tristan da Cunha, during which he spent four weeks on Nightingale Island. On the main island he found that bird stocks were being depleted and the islanders taking too many eggs and young; on Nightingale, however, where there are over two million pairs of great shearwaters, the harvest of these birds could be greater. Inaccessible Island, which like Nightingale, is without cats, dogs or rats, should be declared a wildlife sanctuary. Tl^HEN the first permanent settlers came to Tristan da Cunha in " the early years of the nineteenth century they found an island rich in bird and sea mammal life. "The mountains are covered with Albatross Mellahs Petrels Seahens, etc.," wrote Jonathan Lambert in 1811, and Midshipman Greene, who stayed on the island in 1816, recorded in his diary "Sea Elephants herding together in immense numbers." Today the picture is greatly changed. A century and a half of human habitation has drastically reduced the larger, edible species, and the accidental introduction of rats from a shipwreck in 1882 accelerated the birds' decline on the main island. Wood-cutting, grazing by domestic stock and, more recently, fumes from the volcano have destroyed much of the natural vegetation near the settlement, and two bird subspecies, a bunting and a flightless moorhen, have become extinct on the main island. Curiously, one is liable to see more birds on the day of arrival than in several weeks ashore. When I first saw Tristan from the decks of M.V. -

University of Cape Town
The effects of introduced mice on seabirds breeding at sub-Antarctic Islands Ben J. Dilley Thesis presented for the degree of Doctor of Philosophy Town FitzPatrick Institute of African Ornithology DST/NRF Centre of Excellence Department of Biological Sciences, Faculty of Science University of CapeCape Town of June 2018 University Supervised by Professor Peter G. Ryan The copyright of this thesis vests in the author. No quotation from it or information derivedTown from it is to be published without full acknowledgement of the source. The thesis is to be used for private study or non- commercial research purposes Capeonly. of Published by the University of Cape Town (UCT) in terms of the non-exclusive license granted to UCT by the author. University Declaration This thesis reports original research that I conducted under the auspices of the FitzPatrick Institute, University of Cape Town. All assistance received has been fully acknowledged. This work has not been submitted in any form for a degree at another university. ………………….................. Ben J. Dilley Cape Town, June 2018 i A 10 day-old great shearwater Ardenna gravis chick being attacked by an invasive House mouse Mus musculus in an underground burrow on Gough Island in 2014 (photo Ben Dilley). ii Table of Contents Page Abstract ....................................................................................................................................... iv Acknowledgements .......................................................................................................................... vi Chapter 1 General introduction: Islands, mice and seabirds ......................................................... 1 Chapter 2 Clustered or dispersed: testing the effect of sampling strategy to census burrow-nesting petrels with varied distributions at sub-Antarctic Marion Island ...... 13 Chapter 3 Modest increases in densities of burrow-nesting petrels following the removal of cats Felis catus from sub-Antarctic Marion Island ................................... -

Iucn Red Data List Information on Species Listed On, and Covered by Cms Appendices
UNEP/CMS/ScC-SC4/Doc.8/Rev.1/Annex 1 ANNEX 1 IUCN RED DATA LIST INFORMATION ON SPECIES LISTED ON, AND COVERED BY CMS APPENDICES Content General Information ................................................................................................................................................................................................................................ 2 Species in Appendix I ............................................................................................................................................................................................................................... 3 Mammalia ............................................................................................................................................................................................................................................ 4 Aves ...................................................................................................................................................................................................................................................... 7 Reptilia ............................................................................................................................................................................................................................................... 12 Pisces ................................................................................................................................................................................................................................................. -

Grey Petrels Returning to Campbell Island? Survey and Census 14 Years After Rodent Eradication
Parker et al. 2015 Are grey petrels returning to Campbell Island? Survey and census 14 years after rodent eradication Graham C. Parker, Kalinka Rexer-Huber, David Thompson Report to the Department of Conservation June 2015 __________________________________________________________________________________ Grey petrels, Campbell Island 1 Parker et al. 2015 Are grey petrels returning to Campbell Island? Survey and census 14 years after rodent eradication Report to the Department of Conservation Graham C. Parker 1*, Kalinka Rexer-Huber 1, 3, David Thompson 2 1 Parker Conservation, 126 Maryhill Terrace, Dunedin New Zealand 2 National Institute for Water and Atmosphere (NIWA), 301 Evans Bay Parade, Hataitai, Wellington New Zealand 3 Department of Zoology, University of Otago, 340 Great King Street, Dunedin New Zealand * Author for correspondence: [email protected] Please cite as: Parker, G.C., Rexer-Huber, K. and Thompson, D. 2015. Are grey petrels returning to Campbell Island? Survey and census 14 years after rodent eradication. Unpublished report to the Department of Conservation. Parker Conservation, Dunedin __________________________________________________________________________________ Grey petrels, Campbell Island 2 Parker et al. 2015 Contents Abstract ...................................................................................................................................... 3 Introduction ............................................................................................................................... -

Evaluating Threats to New Zealand Seabirds Report for the Department of Conservation
Evaluating threats to New Zealand seabirds Report for the Department of Conservation Authors: Edward Abraham Yvan Richard Katherine Clements PO Box 27535, Wellington 6141 New Zealand dragonfly.co.nz Cover Notes To be cited as: Abraham, Edward; Yvan Richard; Katherine Clements (2016). Evaluating threats to New Zealand seabirds, 19 pages. Report for the Department of Conservation. Crown copyright © This report is licensed for re-use under a Creative Commons Aribution 3.0 New Zealand Licence. This allows you to distribute, use, and build upon this work, provided credit is given to the original source. Cover image: hps://www.flickr.com/photos/4nitsirk/16121373851 EXECUTIVE SUMMARY The New Zealand Department of Conservation is developing a seabird threat framework, “to beer understand, and manage, at-sea threats to our seabirds”. This framework will allow the impact of threats on seabird populations to be qualitatively assessed, and will be used to prioritise a programme of seabird population monitoring. As a first stage in developing the framework, a database of demographic parameters and threats was prepared. In this project, a process was estab- lished for reviewing and synthesising this information. The demographic parameters were then used to develop an online tool, which allowed for the impact of changes in parameters on population growth rates to be assessed. In the future, this tool will allow the impact of current and potential threats on seabird populations to be promptly explored. The process was trialled on the 12 albatross taxa recognised -

Procellaria Cinerea) on Antipodes Island, New Zealand
269 Notornis, 2013, Vol. 60: 269-278 0029-4470 © The Ornithological Society of New Zealand, Inc. Notes on the distribution, behaviour and status of grey petrel (Procellaria cinerea) on Antipodes Island, New Zealand ELIZABETH. A. BELL* PO Box 607, Blenheim, Marlborough 7240, New Zealand BRIAN D. BELL 35 Selmes Road, Rapaura, RD3, Blenheim 7273, New Zealand J. L. SIM 178 C.D. Farm Road, Ohau, New Zealand M.J. IMBER** 6 Hillcrest Lane, Levin 5500, New Zealand Abstract Aspects of the breeding biology of the grey petrel (Procellaria cinerea) were studied on Antipodes Island between April and June 2001. The island was surveyed to determine grey petrel distribution and four 2500 m2 census grids were established. The survey suggested that the distribution of grey petrels was restricted to steep, well-draining areas dominated by Poa litorosa tussock (approximately 510 ha of the 2025 ha island). Occupied burrow density within the 4 census grids ranged from 31 to 44 burrows (0.01 burrows per square metre). Extrapolating from the census grid density to the total grey petrel habitat resulted in a population estimate of 114,730 birds: 53,000 breeding pairs (range = 32,000- 73,000) and 8,670 non-breeding-birds (range = 4,000-16,320) were present on Antipodes Island. Aspects of the behaviour of the species were recorded. Comparisons are made with other members of the genus Procellaria. Bell, E.A.; Bell, B.D.; Sim, J.L.; Imber, M.J. 2013. Notes on the distribution, behaviour and status of grey petrel (Procellaria cinerea) on Antipodes Island, New Zealand. -
Grey Petrel Procellaria Cinerea (Entire Population) in Appendix II of the Convention on the Conservation of Migratory Species of Wild Animals
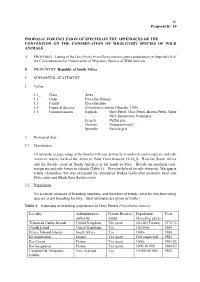
81 Proposal II / 10 PROPOSAL FOR INCLUSION OF SPECIES ON THE APPENDICES OF THE CONVENTION ON THE CONSERVATION OF MIGRATORY SPECIES OF WILD ANIMALS A. PROPOSAL: Listing of the Grey Petrel Procellaria cinerea (entire population) in Appendix II of the Convention on the Conservation of Migratory Species of Wild Animals. B. PROPONENT: Republic of South Africa. C. SUPPORTING STATEMENT 1. Taxon 1.1_ Class Aves 1.2 Order Procellariiformes 1.3 Family Procellariidae 1.4 Genus & Species Procellaria cinerea (Gmelin, 1789) 1.5 Common names English: Grey Petrel, Gray Petrel, Brown Petrel, Great Grey Shearwater, Pediunker French: Puffin gris German: Grausturmvogel Spanish: Pardela gris 2. Biological data 2.1 Distribution Circumpolar pelagic range in the Southern Ocean, primarily in southern cool-temperate and sub- Antarctic waters north of the Antarctic Polar Front between 32-58S. Reaches South Africa and the Pacific coast of South America as far north as Peru. Breeds on southern cool- temperate and sub-Antarctic islands (Table 1). Previously bred on sub-Antarctic Macquarie Island (Australia), but was extirpated by introduced Wekas Gallirallus australis, feral cats Felis catus and Black Rats Rattus rattus. 2.2 Population No accurate censuses of breeding numbers, and therefore of trends, exist for this burrowing species at any breeding locality. Best estimates are given in Table 1. Table 1. Estimates of breeding populations of Grey Petrels Procellaria cinerea Locality Administrative Nature Reserve Population Year authority status (breeding pairs) Tristan da Cunha Islands United Kingdom Yes (part) 50-100 (Tristan) 1972/74 Gough Island United Kingdom Yes 100 000s 1984 Prince Edward Islands South Africa Yes 1000s 1984 Ile Amsterdam France Yes (part) Few suspected 1983 Iles Crozet France Yes (part) 1000s 1981/82 Iles Kerguelen France Yes (part) 5000-10 000 1984-87 Campbell & Antipodes New Zealand Yes 10 000-50 000 1984 Islands 82 Proposal II / 10 2.3 Habitat Marine, in southern coastal and pelagic waters. -

Action Plan for Seabird Conservation in New Zealand Part B: Non-Threatened Seabirds
Action Plan for Seabird Conservation in New Zealand Part B: Non-Threatened Seabirds THREATENED SPECIES OCCASIONAL PUBLICATION NO. 17 Action Plan for Seabird Conservation in New Zealand Part B: Non-Threatened Seabirds THREATENED SPECIES OCCASIONAL PUBLICATION NO. 17 by Graeme A. Taylor Published by Biodiversity Recovery Unit Department of Conservation PO Box 10-420 Wellington New Zealand Illustrations Front cover: Northern diving petrel, North Brothers Island, 1998 Inside front cover: Brown skua, Campbell Island, 1986 Source of illustrations All photographs were taken by the author unless stated otherwise. © May 2000, Department of Conservation ISSN 1170-3709 ISBN 0-478-21925-3 Cataloguing in Publication Taylor, Graeme A. Action plan for seabird conservation in New Zealand. Part B, Non-threatened seabirds / by Graeme A. Taylor. Wellington, N.Z. : Dept. of Conservation, Biodiversity Recovery Unit, 2000. 1. v. ; 30 cm. (Threatened Species occasional publication, 1170-3709 ; 17.) Cataloguing-in-Publication data. - Includes bibliographical references. ISBN 0478219253 1. Sea birds— New Zealand. 2. Rare birds—New Zealand. I. New Zealand. Biodiversity Recovery Unit. II. Title. Series: Threatened species occasional publication ; 17. 236 CONTENTS PART A: THREATENED SEABIRDS Abbreviations used in Parts A and B 7 Abstract 9 1 Purpose 11 2 Scope and limitations 12 3 Sources of information 12 4 General introduction to seabirds 13 4.1 Characteristics of seabirds 14 4.2 Ecology of seabirds 14 4.3 Life history traits of seabirds 15 5 New Zealand seabirds -

Beach Patrol Scheme 2014 - Preliminary Report
BEACH PATROL SCHEME 2014 - PRELIMINARY REPORT This report is based on 231 cards for 2014 received as at 31.12.15. (There were 262 in 2013, 324 in 2012, 352 in 2011, 315 in 2010, 385 in 2009, 381 in 2008 and 403 in 2007). This shows a continuing decline in the number of beach patrols carried out. In 2014 1,069 kilometres were patrolled (1,388km in 2013, 1,625km in 2012, 1,846km in 2011, 3,210 in 2010, 4,470km in 2009, 2,524km in 2008 and 2,307 in 2007). Dead seabirds found in 2014 totalled 1,388 (4,851 in 2013, 1,924 in 2012, 57,920 in 2011, 3,266 in 2010, 4,763 in 2009, 3,906 in 2008, 3,786 in 2007 and 3,117 in 2006). There were no significant wrecks during the year and no especially unusual records. The largest wreck of a single species was of 80 Mottled petrels on Mason Bay on 31/12. The tail end of the Short-tailed shearwater wreck of late 2013 was still being seen in early 2014. The recovery of a Red-legged partridge was probably a first for the beach patrol scheme. The average recovery rate for the whole country is around 1.33 birds per kilometre compared with the 2013 average of 3.65, largely influenced by a significant wreck of juvenile Sooty shearwaters in that year. The 2012 average was 1.8 birds and the 2011 average of 31 birds per kilometre was boosted by the huge prion wreck that year. -

Population Size and Trends of Four Globally Threatened Seabirds at Gough Island, South Atlantic Ocean
97 POPULATION SIZE AND TRENDS OF FOUR GLOBALLY THREATENED SEABIRDS AT GOUGH ISLAND, SOUTH ATLANTIC OCEAN RICHARD J. CUTHBERT1 & ERICA S. SOMMER 1Royal Society for the Protection of Birds, The Lodge, Sandy, Bedfordshire, SG19 2DL, UK and Percy FitzPatrick Institute of African Ornithology, University of Cape Town, Rondebosch 7701, South Africa ([email protected]) Current address: Beacon Ecology, Vine Cottage, Middletown, Hailey, Witney, Oxfordshire, OX29 9UB, UK Received 17 June 2003, accepted 4 November 2003 SUMMARY CUTHBERT, R.J. & SOMMER, E.S. 2004. Population size and trends of four globally threatened seabirds at Gough Island, South Atlantic Ocean. Marine Ornithology 32: 97-103. Gough Island in the South Atlantic Ocean is a site of international significance for breeding seabirds, yet for most species population estimates have not been quantified and there is as yet no formal provision for long-term monitoring. This study undertook surveys of four species of globally threatened seabirds: Northern Rockhopper Penguins Eudyptes chrysocome moseleyi (Vulnerable), Atlantic Yellow-nosed Albatrosses Thalassarche chlororhynchos (Endangered), Sooty Albatrosses Phoebetria fusca (Endangered) and Southern Giant Petrels Macronectes giganteus (Vulnerable) from September 2000 to September 2001. Northern Rockhopper Penguins were only counted from a limited number of sites. However, comparison of numbers at The Glen and Sophora Glen in 2000 with earlier counts at these two sites suggests that the island population is still around 145 000 pairs. Ground counts of 11% of available habitat indicate that Atlantic Yellow- nosed Albatrosses nest at an average density of 5.0±1.0 pairs/ha, and the total breeding population is estimated to be 5300 pairs (CI: 3000 to 7500 pairs). -

Analysis of Albatross and Petrel Distribution Within the CCAMLR Convention Area: Results from the Global Procellariiform Tracking Database
CCAMLR Science, Vol. 13 (2006): 143–174 ANALYSIS OF ALBATROSS AND PETREL DISTRIBUTION WITHIN THE CCAMLR CONVENTION AREA: RESULTS FROM THE GLOBAL PROCELLARIIFORM TRACKING DATABASE BirdLife International (prepared by C. Small and F. Taylor) Wellbrook Court, Girton Road Cambridge CB3 0NA United Kingdom Correspondence address: BirdLife Global Seabird Programme c/o Royal Society for the Protection of Birds The Lodge, Sandy Bedfordshire SG19 2DL United Kingdom Email – [email protected] Abstract CCAMLR has implemented successful measures to reduce the incidental mortality of seabirds in most of the fisheries within its jurisdiction, and has done so through area- specific risk assessments linked to mandatory use of measures to reduce or eliminate incidental mortality, as well as through measures aimed at reducing illegal, unreported and unregulated (IUU) fishing. This paper presents an analysis of the distribution of albatrosses and petrels in the CCAMLR Convention Area to inform the CCAMLR risk- assessment process. Albatross and petrel distribution is analysed in terms of its division into FAO areas, subareas, divisions and subdivisions, based on remote-tracking data contributed to the Global Procellariiform Tracking Database by multiple data holders. The results highlight the importance of the Convention Area, particularly for breeding populations of wandering, grey-headed, light-mantled, black-browed and sooty albatross, and populations of northern and southern giant petrel, white-chinned petrel and short- tailed shearwater. Overall, the subareas with the highest proportion of albatross and petrel breeding distribution were Subareas 48.3 and 58.6, adjacent to the southwest Atlantic Ocean and southwest Indian Ocean, but albatross and petrel breeding ranges extend across the majority of the Convention Area. -

Sherbornia Date of Publication: an Open-Access Journal of Bibliographic 29 March 2021 and Nomenclatural Research in Zoology
E-ISSN 2373-7697 Volume 7(1): 1–4 Sherbornia Date of Publication: An Open-Access Journal of Bibliographic 29 March 2021 and Nomenclatural Research in Zoology Research Note The source of the name of the Sooty Albatross, Phoebetria fusca: a correction Murray D. Bruce P.O. Box 180, Turramurra 2074, NSW, Australia; [email protected] lsid:zoobank.org:pub: 442B27EBF0794BE58FBB65D71B8EB8C8 Karl [or Carl] Theodor Hilsenberg (1802–1824) 787)2. Moreover, another Hilsenbergia already was a young naturalist and collector, principally had been named (Reichenbach 1828: 117) and as a botanist, who died at sea off Île Sainte although later synonymised with Ehretia L., it is Marie (Nosy Boraha), an island off the northeast now recognised as a distinct genus, comprising coast of Madagascar on 11 September 1824 at 21 species in the forgetmenot family Boragi only 22 years of age (Pritzel 1872: 144; Hooker, naceae3. in Hilsenberg & Bojer 1833: 246–247). He had More recently, Hilsenberg’s claim to fame, travelled to Mauritius with his friend and fellow and actually published while he was still active, botanist Wenceslas Bojer (1795–1856), arriving was in naming a new species of albatross, there on 6 July 1821. After spending about a Diomedea fusca (Hilsenberg 1822a: col. 1164). year in Madagascar in 1822–1823, Hilsenberg A short note on his Diomedea fusca, including and Bojer were back at Mauritius. It was during circumscriptive details, also was provided in a a second expedition, departing in 1824, on its periodical usually referred to as ‘Froriep’s Noti- way to the eastern coast of Africa, with Bojer, zen’.