NIH Public Access Author Manuscript Neuroimage
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans Ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai
Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai To cite this version: Hans ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai. Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex. Frontiers in Neuroanatomy, Frontiers, 2018, 12, pp.93. 10.3389/fnana.2018.00093. hal-01929541 HAL Id: hal-01929541 https://hal.archives-ouvertes.fr/hal-01929541 Submitted on 21 Nov 2018 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. REVIEW published: 19 November 2018 doi: 10.3389/fnana.2018.00093 Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans J. ten Donkelaar 1*†, Nathalie Tzourio-Mazoyer 2† and Jürgen K. Mai 3† 1 Department of Neurology, Donders Center for Medical Neuroscience, Radboud University Medical Center, Nijmegen, Netherlands, 2 IMN Institut des Maladies Neurodégénératives UMR 5293, Université de Bordeaux, Bordeaux, France, 3 Institute for Anatomy, Heinrich Heine University, Düsseldorf, Germany The gyri and sulci of the human brain were defined by pioneers such as Louis-Pierre Gratiolet and Alexander Ecker, and extensified by, among others, Dejerine (1895) and von Economo and Koskinas (1925). -

Occipital Sulci Patterns in Patients with Schizophrenia and Migraine Headache Using Magnetic Resonance Imaging (MRI)
ORIGINAL ARTICLE Occipital sulci patterns in patients with schizophrenia and migraine headache using magnetic resonance imaging (MRI) Gorana Sulejmanpašić1, Enra Suljić2, Selma Šabanagić-Hajrić2 1Department of Psychiatry, 2Department of Neurology; University Clinical Center Sarajevo, Sarajevo, Bosnia and Herzegovina ABSTRACT Aim To examine the presence of morphologic variations of occi- pital sulci patternsin patients with schizophrenia and migraine he- adacheregarding gender and laterality using magnetic resonance imaging (MRI). MethodsThis study included 80 patients and brain scans were performed to analyze interhemispheric symmetry and the sulcal patterns of the occipital region of both hemispheres. Average total volumes of both hemispheres of the healthy population were used for comparison. Results There was statistically significant difference between su- bjects considering gender (p=0.012)with no difference regarding Corresponding author: age(p=0.1821). Parameters of parieto-occipital fissure (p=0.0314), Gorana Sulejmanpašić body of the calcarine sulcus (p=0.0213), inferior sagittal sulcus (p=0.0443), and lateral occipital sulcus (p=0.0411) showed stati- Department of Psychiatry, Clinical Center stically significant difference only of left hemisphere in male pati- University of Sarajevo ents with schizophrenia with shallowerdepth of the sulcus. Bolnička 25, 71000 Sarajevo, Bosnia and Herzegovina Conclusion Representation of neuroanatomical structures sugge- sts the existence of structural neuroanatomic disorders with focal Phone: +387 33 29 75 38; brain changes. Comparative analysis of occipital lobe and their Fax:+387 33 29 85 23; morphologic structures (cortical dysmorphology) in patients with E mail: [email protected] schizophreniausing MRI, according to genderindicates a signifi- cant cortical reduction in the left hemisphere only in the group of male patients compared to female patients and the control group. -

PDF Hosted at the Radboud Repository of the Radboud University Nijmegen
PDF hosted at the Radboud Repository of the Radboud University Nijmegen The following full text is a publisher's version. For additional information about this publication click this link. http://hdl.handle.net/2066/200480 Please be advised that this information was generated on 2021-10-05 and may be subject to change. REVIEW published: 19 November 2018 doi: 10.3389/fnana.2018.00093 Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans J. ten Donkelaar 1*†, Nathalie Tzourio-Mazoyer 2† and Jürgen K. Mai 3† 1 Department of Neurology, Donders Center for Medical Neuroscience, Radboud University Medical Center, Nijmegen, Netherlands, 2 IMN Institut des Maladies Neurodégénératives UMR 5293, Université de Bordeaux, Bordeaux, France, 3 Institute for Anatomy, Heinrich Heine University, Düsseldorf, Germany The gyri and sulci of the human brain were defined by pioneers such as Louis-Pierre Gratiolet and Alexander Ecker, and extensified by, among others, Dejerine (1895) and von Economo and Koskinas (1925). Extensive discussions of the cerebral sulci and their variations were presented by Ono et al. (1990), Duvernoy (1992), Tamraz and Comair (2000), and Rhoton (2007). An anatomical parcellation of the spatially normalized single high resolution T1 volume provided by the Montreal Neurological Institute (MNI; Collins, 1994; Collins et al., 1998) was used for the macroscopical labeling of functional studies (Tzourio-Mazoyer et al., 2002; Rolls et al., 2015). In the standard atlas of the human brain by Mai et al. (2016), the terminology from Mai and Paxinos (2012) is used. It contains an extensively analyzed individual brain hemisphere in the MNI- space. -

Complementary Patterns of Direct Amygdala and Hippocampal Projections to the Macaque Prefrontal Cortex John P
Cerebral Cortex, November 2015;25: 4351–4373 doi: 10.1093/cercor/bhv019 Advance Access Publication Date: 24 February 2015 Original Article ORIGINAL ARTICLE Complementary Patterns of Direct Amygdala and Hippocampal Projections to the Macaque Prefrontal Cortex John P. Aggleton1, Nicholas F. Wright1, Douglas L. Rosene2, and Richard C. Saunders3 1School of Psychology, Cardiff University, Cardiff, Wales CF10 3AT, UK, 2School of Medicine, Department of Anatomy and Neurobiology, Boston University, Boston MA 02118, USA, and 3Laboratory of Neuropsychology, National Institute of Mental Health, Bethesda, MD 20892, USA Address correspondence to: John P. Aggleton, School of Psychology, Cardiff University, Park Place, Cardiff, Wales CF10 3AT, UK. Email: [email protected] Abstract The projections from the amygdala and hippocampus (including subiculum and presubiculum) to prefrontal cortex were compared using anterograde tracers injected into macaque monkeys (Macaca fascicularis, Macaca mulatta). Almost all prefrontal areas were found to receive some amygdala inputs. These connections, which predominantly arose from the intermediate and magnocellular basal nucleus, were particularly dense in parts of the medial and orbital prefrontal cortex. Contralateral inputs were not, however, observed. The hippocampal projections to prefrontal areas were far more restricted, being confined to the ipsilateral medial and orbital prefrontal cortex (within areas 11, 13, 14, 24a, 32, and 25). These hippocampal projections principally arose from the subiculum, with the fornix providing the sole route. Thus, while the lateral prefrontal cortex essentially receives only amygdala inputs, the orbital prefrontal cortex receives both amygdala and hippocampal inputs, though these typically target different areas. Only in medial prefrontal cortex do direct inputs from both structures terminate in common sites. -

Cortical Parcellation Protocol
CORTICAL PARCELLATION PROTOCOL APRIL 5, 2010 © 2010 NEUROMORPHOMETRICS, INC. ALL RIGHTS RESERVED. PRINCIPAL AUTHORS: Jason Tourville, Ph.D. Research Assistant Professor Department of Cognitive and Neural Systems Boston University Ruth Carper, Ph.D. Assistant Research Scientist Center for Human Development University of California, San Diego Georges Salamon, M.D. Research Dept., Radiology David Geffen School of Medicine at UCLA WITH CONTRIBUTIONS FROM MANY OTHERS Neuromorphometrics, Inc. 22 Westminster Street Somerville MA, 02144-1630 Phone/Fax (617) 776-7844 neuromorphometrics.com OVERVIEW The cerebral cortex is divided into 49 macro-anatomically defined regions in each hemisphere that are of broad interest to the neuroimaging community. Region of interest (ROI) boundary definitions were derived from a number of cortical labeling methods currently in use. Protocols from the Laboratory of Neuroimaging at UCLA (LONI; Shattuck et al., 2008), the University of Iowa Mental Health Clinical Research Center (IOWA; Crespo-Facorro et al., 2000; Kim et al., 2000), the Center for Morphometric Analysis at Massachusetts General Hospital (MGH-CMA; Caviness et al., 1996), a collaboration between the Freesurfer group at MGH and Boston University School of Medicine (MGH-Desikan; Desikan et al., 2006), and UC San Diego (Carper & Courchesne, 2000; Carper & Courchesne, 2005; Carper et al., 2002) are specifically referenced in the protocol below. Methods developed at Boston University (Tourville & Guenther, 2003), Brigham and Women’s Hospital (McCarley & Shenton, 2008), Stanford (Allan Reiss lab), the University of Maryland (Buchanan et al., 2004), and the University of Toyoma (Zhou et al., 2007) were also consulted. The development of the protocol was also guided by the Ono, Kubik, and Abernathy (1990), Duvernoy (1999), and Mai, Paxinos, and Voss (Mai et al., 2008) neuroanatomical atlases. -
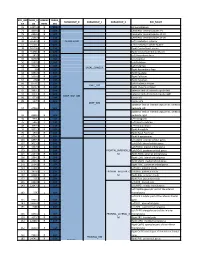
Core-Example1.Pdf
ROI_IND NUM_V HEMISP TISSUE_ SUBGROUP_0 SUBGROUP_1 SUBGROUP_2 ROI_NAME EX OX HERE SEG 95 12871.8 B WM corpus callosum 71 4899.8 B GM Cerebellar Vermal Lobules I-V 73 2858.8 B GM Cerebellar Vermal Lobules VIII-X 72 2266.9 B GM Cerebellar Vermal Lobules VI-VII 39 54582.6 L GM CEREBELLUM Left Cerebellum Exterior 41 15500.7 L WM Left Cerebellum White Matter 38 54379.4 R GM Right Cerebellum Exterior 40 15458.7 R WM Right Cerebellum White Matter 30 585.9 L GM Left Accumbens Area 37 3578.9 L GM Left Caudate 56 1597.6 L GM Left Pallidum 58 4942.3 L GM Left Putamen BASAL_GANGLIA 23 526 R GM Right Accumbens Area 36 3651.5 R GM Right Caudate 55 1638.8 R GM Right Pallidum 57 4726 R GM Right Putamen 60 8574.1 L GM Left Thalamus Proper DEEP_GM 59 8256.3 R GM Right Thalamus Proper 92 2887.7 L WM anterior limb of internal capsule left 91 3393.3 R WM anterior limb of internal capsule right DEEP_WM_GM 90 673.6 L WM fornix left 89 517.5 R WM fornix right DEEP_WM posterior limb of internal capsule inc. cerebral 94 2416.3 L WM peduncle left posterior limb of internal capsule inc. cerebral 93 2480.5 R WM peduncle right 32 993.7 L GM Left Amygdala 75 586.5 L GM Left Basal Forebrain 48 3597.7 L GM Left Hippocampus 31 1021.3 R GM Right Amygdala 76 593.1 R GM Right Basal Forebrain 47 3704.7 R GM Right Hippocampus 105 1897.7 L GM Left AOrG anterior orbital gyrus 137 3015.9 L GM Left LOrG lateral orbital gyrus 147 4637.3 L GM Left MOrG medial orbital gyrus 179 2915.7 L GM FRONTAL_INFERIOR_G Left POrG posterior orbital gyrus 104 2244.9 R GM M Right AOrG anterior orbital -

Supplemental Data
Supplemental Figure 1. Representative clinical 18F-FDG brain PET images from two extremes of image evaluation. The images shown are (A) low-dose, (B) deep learning prediction in image space, (C) deep learning prediction in sinogram space, (D) full-dose. The red numbers indicate scores assigned by one expert physician to images. THE JOURNAL OF NUCLEAR MEDICINE • Vol. 61• No. 9 • September 2020 Sanaat et al. STD of SUV bias SUV bias(%) White matter Third ventricle Temporal horn Frontal Horn Brainstem Cerebellum Cingulate gyrus, pos Cingulate gyrus, ant Insula Substantia nigra Corp Callosum Pallidum Thalamus Putamen Nucleus accumbens Caudate nucleus Cuneus Lingual gyrus Lat occipital lobe Inf parietal lobe Superior parietal gyrus Anterior Sup Temporal Gyrus Posterior temporal lobe Fusiform Mid & Inf temporal gyrus Superior temporal gyrus Para hippocampal Temporal lobe, lateral Temporal lobe, medial Amygdala Hippocampus Subgenual frontal Subcallosal area Subgenual frontal cortex Posterior orbital gyrus Lateral orbital gyrus Medial orbital gyrus Superior frontal gyrus Inferior frontal gyrus Anterior orbital gyrus Straight gyrus Precentral gyrus Middlle frontal gyrus 0 0.5 1 1.5 2 -3 -1 1 3 LD PIS PSS LD PIS PSS THE JOURNAL OF NUCLEAR MEDICINE • Vol. 61• No. 9 • September 2020 Sanaat et al. Supplemental Figure 2. Plots of SUV bias standard deviation (left panel) and mean SUV bias (%) (right panel) in the different brain regions for LD, PIS, and PSS PET images. The left and right regions were merged, thus reducing the number of brain regions to 44. THE JOURNAL OF NUCLEAR MEDICINE • Vol. 61• No. 9 • September 2020 Sanaat et al. THE JOURNAL OF NUCLEAR MEDICINE • Vol. -

Anatomic Moment Limbic System Anatomy: an Overview
Anatomic Moment Limbic System Anatomy: An Overview Leighton P. Mark,1.3 David L. Daniels, 1 Thomas P. Naidich,2 and Jessica A. Borne1 The anatomy of the limbic system has become shaped sulcus (Fig. 2) designated as 1) the hip more relevant due to the development of high pocampal fissure where it lies superior to the resolution and functional magnetic resonance parahippocampal gyrus, and 2) the callosal sulcus (MR) imaging. This review is the first of a series where it lies inferior to the cingulate gyrus of Anatomic Moments designed to highlight se (Fig. 3). lected features of limbic system anatomy in order Nested within this )-shaped sulcus is another ) to facilitate their application to MR interpretation. shaped structure formed by 1) the dentate gyrus Limbic system terminology of early anatomists and hippocampus in the temporal lobe (Figs. 2 was largely descriptive (Table 1), but the nomen and 4), 2) the hippocampal tail which consists of clature used in this series will follow that of recent thin gray and white matter structures located just authors (Table 2) (1-7). posterior and inferior to the splenium of the In accordance with the curvilinear development corpus callosum (Fig. 5), and 3) the supracallosal of the cerebral hemispheres (Fig. 1), the struc gyrus which is located inferior to the callosal tures of the limbic lobe form a series of "nested" sulcus. The supracallosal gyrus is intimately ap )-shaped curves (Fig. 2). plied to the upper surface of the corpus callosum, The widest curve is formed by the large limbic and contains gray matter termed the indusium gyrus which is designated as 1) the parahippo griseum (Fig. -

Mapping Striate and Extrastriate Visual Areas in Human Cerebral Cortex EDGAR A
Proc. Natl. Acad. Sci. USA Vol. 93, pp. 2382-2386, March 1996 Neurobiology Mapping striate and extrastriate visual areas in human cerebral cortex EDGAR A. DEYOE*, GEORGE J. CARMANt, PETER BANDETTINIt, SETH GLICKMAN*, JON WIESER*, ROBERT COX§, DAVID MILLER1, AND JAY NEITZ* *Department of Cellular Biology and Anatomy, and Biophysics Research Institute, The Medical College of Wisconsin, 8701 Watertown Plank Road, Milwaukee, WI 53226; tThe Salk Institute for Biological Studies, La Jolla, CA 92037; tMassachusetts General Hospital-NMR Center, Charlestown, MA 02129; §Biophysics Research Institute, The Medical College of Wisconsin, Milwaukee, WI 53226; and lBrown University, Providence, RI 02912 Communicated by Francis Crick The Salk Institute for Biological Sciences, San Diego, CA, November 14, 1995 (received for review August 2, 1995) ABSTRACT Functional magnetic resonance imaging represented by each active site in the brain (12). A similar (fMRI) was used to identify and map the representation of the technique has been described by Engel et al. (15). visual field in seven areas of human cerebral cortex and to To enhance activation of extrastriate cortex (12) and to help identify at least two additional visually responsive regions. maintain attention and arousal, the subject was required to The cortical locations of neurons responding to stimulation detect a small target appearing at a random position super- along the vertical or horizontal visual field meridia were imposed on the checkered hemifield or annulus. A new target charted on three-dimensional models of the cortex and on was presented every 2 sec and the subject activated one of two unfolded maps of the cortical surface. -

Human Brain – 4 Part 1313104
V1/17-2016© Human Brain – 4 Part 1313104 Left Hemisphere of Cerebrum, Lateral View 1. 37. Cingulate Gyrus a. Motor Area (Somatomotor Cortex) 38. Cingulate Sulcus b. Sensory Area (Somatosensory Cortex) 39. Subfrontal Part of Cingulate Sulcus 2. Frontal Lobe 40. Sulcus of Corpus Callosum 3. Temporal Lobe 41. Body of Corpus Callosum 4. Parietal Lobe 42. Genu of Corpus Callosum 5. Occipital Lobe 43. Splenium of Corpus Callosum 6. Left Hemisphere of Cerebellum 44. Septum Pellucidum 7. Superior Frontal Gyrus 45. Body of Fornix 8. Middle Frontal Gyrus 46. Anterior Commissure 9. Inferior Frontal Gyrus 47. Intermediate Mass 10. Precentral Gyrus (Somatomotor Cortex) 48. Posterior Commissure 11. Postcentral Gyrus (Somatosensory Cortex) 49. Thalamus 12. Superior Parietal Lobule 50. Foramen of Monro 13. Inferior Parietal Lobule 51. Hypothalamic Sulcus 14. Lateral Occipital Gyri 52. Lamina Terminalis 15. Superior Temporal Gyrus 53. Optic Chiasm 16. Middle Temporal Gyrus 54. Pineal Gland 17. Inferior Temporal Gyrus 55. Pineal Recess 18. Superior Frontal Sulcus 56. Corpora Quadrigemina 19. Pre-central Sulcus 57. Sylvian Aqueduct 20. Central Sulcus 58. Superior Medullary Vellum 21. Post-central Sulcus 59. Fourth Ventricle 22. Intraparietal Sulcus 60. Central Canal of Medulla Oblongata 23. Transverse Occipital Sulcus 61. Arbor Vitae 24. Lateral Occipital Sulcus 62. Vermis of Cerebellum 25. Parieto-occipital Sulcus 63. Medulla Oblongata 26. Superior Temporal Sulcus 64. Medial Longitudinal Fasciculus 27. Middle Temporal Sulcus 65. Pons 28. Lateral Cerebral Sulcus (Sylvian Fissure) 66. Longitudinal Fibers of Pons 29. Anterior Horizontal Ramus of Sylvian Fissure 67. Cerebral Peduncle 30. Anterior Ascending Ramus of Sylvian Fissure 68. Tuber Cinereum 31. -

Two Retinotopic Visual Areas in Human Lateral Occipital Cortex
13128 • The Journal of Neuroscience, December 20, 2006 • 26(51):13128–13142 Behavioral/Systems/Cognitive Two Retinotopic Visual Areas in Human Lateral Occipital Cortex Jonas Larsson and David J. Heeger Department of Psychology and Center for Neural Science, New York University, New York, New York 10003 We describe two visual field maps, lateral occipital areas 1 (LO1) and 2 (LO2), in the human lateral occipital cortex between the dorsal part of visual area V3 and visual area V5/MTϩ. Each map contained a topographic representation of the contralateral visual hemifield. The eccentricity representations were shared with V1/V2/V3. The polar angle representation in LO1 extended from the lower vertical merid- ian (at the boundary with dorsal V3) through the horizontal to the upper vertical meridian (at the boundary with LO2). The polar angle representationinLO2wasthemirror-reversalofthatinLO1.LO1andLO2overlappedwiththeposteriorpartoftheobject-selectivelateral occipital complex and the kinetic occipital region (KO). The retinotopy and functional properties of LO1 and LO2 suggest that they correspond to two new human visual areas, which lack exact homologues in macaque visual cortex. The topography, stimulus selectivity, and anatomical location of LO1 and LO2 indicate that they integrate shape information from multiple visual submodalities in retinotopic coordinates. Key words: fMRI; homology; human; lateral occipital; retinotopy; V4; visual cortex Introduction and KO are large and functionally heterogeneous cortical regions The primate visual cortex can be subdivided into a number of that are believed to include multiple subregions (Grill-Spector et distinct areas on the basis of retinotopy, functional properties, al., 1999; Hasson et al., 2003; Zeki et al., 2003; Sawamura et al., anatomical connections, and microstructure (Gattass et al., 2005; Tyler et al., 2005a). -

031909.M1-Cns.Limbicsystem.Pdf
Attribution: Department of Neurology, 2009 License: Unless otherwise noted, this material is made available under the terms of the Creative Commons Attribution–Non-commercial–Share Alike 3.0 License: http://creativecommons.org/licenses/by-nc-sa/3.0/ We have reviewed this material in accordance with U.S. Copyright Law and have tried to maximize your ability to use, share, and adapt it. The citation key on the following slide provides information about how you may share and adapt this material. Copyright holders of content included in this material should contact [email protected] with any questions, corrections, or clarification regarding the use of content. For more information about how to cite these materials visit http://open.umich.edu/education/about/terms-of-use. Any medical information in this material is intended to inform and educate and is not a tool for self-diagnosis or a replacement for medical evaluation, advice, diagnosis or treatment by a healthcare professional. Please speak to your physician if you have questions about your medical condition. Viewer discretion is advised: Some medical content is graphic and may not be suitable for all viewers. Citation Key for more information see: http://open.umich.edu/wiki/CitationPolicy Use + Share + Adapt { Content the copyright holder, author, or law permits you to use, share and adapt. } Public Domain – Government: Works that are produced by the U.S. Government. (USC 17 § 105) Public Domain – Expired: Works that are no longer protected due to an expired copyright term. Public Domain – Self Dedicated: Works that a copyright holder has dedicated to the public domain.