Durham E-Theses
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Antagonistic Coevolution Between the Sexes in a Group of Insects
letters to nature Acknowledgements a central process of evolution, with the potential to shape various We thank the authorities in Madagascar and the Seychelles for permission to conduct interactions between the sexes2,5,6 and their gametes7±8, as well as ®eldwork. We thank J. Brown, T. Peterson, R. Prum, O. Rieppel, L. Trueb and E. Wiley for diversi®cation9, speciation and extinction rates10±12. At the core of comments. C.J.R. and R.A.N. were supported by the National Geographic Society and the this coevolutionary interaction is an arms race between the sexes National Science Foundation, Washington. that can include periods of both escalation and de-escalation of arms13±15. Competing interests statement Theory suggests, however, that the outcome of antagonistic The authors declare that they have no competing ®nancial interests. male±female interactions should remain relatively unchanged Correspondence and requests for materials should be addressed to C.J.R. during an arms race because the build-up of arms in one sex may (e-mail: [email protected]). Sequences are deposited of GenBank under accession numbers be balanced by a build-up in the other (Fig. 1a, 1±2). The AF443224±AF443275. consequences of such arms races on sexual interactions may thus be undetectable, which makes sexually antagonistic coevolution inherently dif®cult to show1±4. Perhaps for this reason, we have no direct empirical evidence for a primary role of arms races in the ................................................................. evolution of sexual interactions in natural systems. Despite an expectation of some evolutionary balance in the level Antagonistic coevolution between of arms between the sexes, one sex may at least temporarily evolve a greater quantity of arms relative to the other (refs 13±15; and Fig. -

A Check List of Gerromorpha (Hemiptera) from India
Rec. zool. Surv. India: 100 (Part 1-2) : 55-97, 2002 A CHECK LIST OF GERROMORPHA (HEMIPTERA) FROM INDIA G. THIRUMALAI Zoological Survey of India, Southern Regional Station, Chennai 600 028. INTRODUCflON The Infraorder Gerromorpha comprises of semi-aquatic bugs characterised by long conspicuous antennae, longer than head and inserted in front of eyes. They are distributed in all kinds of climatic zones, except the coldest and driest parts. This infraorder contains 8 families namely, Gerridae, Veliidae, Hydrometridae, Mesoveliidae, Hebridae, Macroveliidae, Paraphrynoveliidae and Hennatobatidae (Andersen, 1982a). Knowledge of Indian semi-aquatic Hemiptera is limited to the taxonomic preliminaries, recording species from different parts of the country. (Distant, 1903a; 1910 a&b; Annandale, 1919; Bergroth, 1915a; Paiva, 1919a & b; Dover, 1928; Hafiz & Mathai, 1938; Hafiz & Riberio, 1939; Hafiz & Pradhan, 1947; Pradhan, 1950a, b& 1975; Gupta, 1981; Selvanayagam, 1981; Roy et al. 1988; Ghosh et al. 1989; Polhenlus & Starmuhlner, 1990; Bal & Basu, 1994 & 1997; Chen & Zettel, 1999). The revisionary work of Andersen (1975, 1980, 1990 & 1993); Den Boer (1969); Hungerford & Matsuda (1958a & b, 1960, 1962b & 1965); Herring (1961); Polhemus & Andersen (1984); Andersen & Foster (1992); Andersen & Chen (1993); Chen & Nieser (1993a & b); Polhemus & Karunaratne (1993); Polhemus (1994); Polhemus & Polhemus (1994 & 1995a) on a few genera of Gerridae; Lundblad (1936) on the genera Rhagovelia Mayr and Tetraripis Lundblad; Andersen (1981 b, 1983 & 1989); Polhemus -

Heteroptera: Gerromorpha) in Central Europe
Shortened web version University of South Bohemia in České Budějovice Faculty of Science Ecology of Veliidae and Mesoveliidae (Heteroptera: Gerromorpha) in Central Europe RNDr. Tomáš Ditrich Ph.D. Thesis Supervisor: Prof. RNDr. Miroslav Papáček, CSc. University of South Bohemia, Faculty of Education České Budějovice 2010 Shortened web version Ditrich, T., 2010: Ecology of Veliidae and Mesoveliidae (Heteroptera: Gerromorpha) in Central Europe. Ph.D. Thesis, in English. – 85 p., Faculty of Science, University of South Bohemia, České Budějovice, Czech Republic. Annotation Ecology of Veliidae and Mesoveliidae (Hemiptera: Heteroptera: Gerromorpha) was studied in selected European species. The research of these non-gerrid semiaquatic bugs was especially focused on voltinism, overwintering with physiological consequences and wing polymorphism with dispersal pattern. Hypotheses based on data from field surveys were tested by laboratory, mesocosm and field experiments. New data on life history traits and their ecophysiological consequences are discussed in seven original research papers (four papers published in peer-reviewed journals, one paper accepted to publication, one submitted paper and one communication in a conference proceedings), creating core of this thesis. Keywords Insects, semiaquatic bugs, life history, overwintering, voltinism, dispersion, wing polymorphism. Financial support This thesis was mainly supported by grant of The Ministry of Education, Youth and Sports of the Czech Republic No. MSM 6007665801, partially by grant of the Grant Agency of the University of South Bohemia No. GAJU 6/2007/P-PřF, by The Research Council of Norway: The YGGDRASIL mobility program No. 195759/V11 and by Czech Science Foundation grant No. 206/07/0269. Shortened web version Declaration I hereby declare that I worked out this Ph.D. -

Sexually Antagonistic Coevolution in a Mating System
Sexually Antagonistic Coevolution in a Mating System: Combining Experimental and Comparative Approaches to Address Evolutionary Processes Author(s): Locke Rowe and Göran Arnqvist Reviewed work(s): Source: Evolution, Vol. 56, No. 4 (Apr., 2002), pp. 754-767 Published by: Society for the Study of Evolution Stable URL: http://www.jstor.org/stable/3061658 . Accessed: 01/10/2012 15:38 Your use of the JSTOR archive indicates your acceptance of the Terms & Conditions of Use, available at . http://www.jstor.org/page/info/about/policies/terms.jsp . JSTOR is a not-for-profit service that helps scholars, researchers, and students discover, use, and build upon a wide range of content in a trusted digital archive. We use information technology and tools to increase productivity and facilitate new forms of scholarship. For more information about JSTOR, please contact [email protected]. Society for the Study of Evolution is collaborating with JSTOR to digitize, preserve and extend access to Evolution. http://www.jstor.org Evoluition, 56(4), 2002, pp. 754-767 SEXUALLY ANTAGONISTIC COEVOLUTION IN A MATING SYSTEM: COMBINING EXPERIMENTAL AND COMPARATIVE APPROACHES TO ADDRESS EVOLUTIONARY PROCESSES LOCKE ROWE"223 AND GORAN ARNQVIST4 'Department of Zoology, University of Toronto, Toronto, Ontario M5S 3G5, Canada 2Centre for Biodiversity and Conservation Biology, Royal Ontario Museum, Toronto, Ontario M5S 2C6 Canada 3E-mail: [email protected] 4Department of Animal Ecology, Evolutionary Biology Centre, University of Uppsala, Norbyvagen 18d, SE-752 36 Uppsala, Sweden Abstract.-We combined experimental and comparative techniques to study the evolution of mating behaviors within in a clade of 15 water striders (Gerris spp.). -

A Comparison of the External Morphology and Functions of Labial Tip Sensilla in Semiaquatic Bugs (Hemiptera: Heteroptera: Gerromorpha)
Eur. J. Entomol. 111(2): 275–297, 2014 doi: 10.14411/eje.2014.033 ISSN 1210-5759 (print), 1802-8829 (online) A comparison of the external morphology and functions of labial tip sensilla in semiaquatic bugs (Hemiptera: Heteroptera: Gerromorpha) 1 2 JOLANTA BROŻeK and HERBERT ZeTTeL 1 Department of Zoology, Faculty of Biology and environmental Protection, University of Silesia, Bankowa 9, PL 40-007 Katowice, Poland; e-mail: [email protected] 2 Natural History Museum, entomological Department, Burgring 7, 1010 Vienna, Austria; e-mail: [email protected] Key words. Heteroptera, Gerromorpha, labial tip sensilla, pattern, morphology, function, apomorphic characters Abstract. The present study provides new data on the morphology and distribution of the labial tip sensilla of 41 species of 20 gerro- morphan (sub)families (Heteroptera: Gerromorpha) obtained using a scanning electron microscope. There are eleven morphologically distinct types of sensilla on the tip of the labium: four types of basiconic uniporous sensilla, two types of plate sensilla, one type of peg uniporous sensilla, peg-in-pit sensilla, dome-shaped sensilla, placoid multiporous sensilla and elongated placoid multiporous sub- apical sensilla. Based on their external structure, it is likely that these sensilla are thermo-hygrosensitive, chemosensitive and mechano- chemosensitive. There are three different designs of sensilla in the Gerromorpha: the basic design occurs in Mesoveliidae and Hebridae; the intermediate one is typical of Hydrometridae and Hermatobatidae, and the most specialized design in Macroveliidae, Veliidae and Gerridae. No new synapomorphies for Gerromorpha were identified in terms of the labial tip sensilla, multi-peg structures and shape of the labial tip, but eleven new diagnostic characters are recorded for clades currently recognized in this infraorder. -

Systematics, Historical Biogeography and Ecological Phylogenetics in A
ZOBODAT - www.zobodat.at Zoologisch-Botanische Datenbank/Zoological-Botanical Database Digitale Literatur/Digital Literature Zeitschrift/Journal: Denisia Jahr/Year: 2006 Band/Volume: 0019 Autor(en)/Author(s): Damgaard Jakob Artikel/Article: Systematics, Historical Biogeography and Ecological Phylogenetics in a clade of water striders 813-822 © Biologiezentrum Linz/Austria; download unter www.biologiezentrum.at Systematics, Historical Biogeography and Ecological Phylogenetics in a clade of water striders1 J. DAMGAARD Abstract: I hereby review the current knowledge about systematics, historical biogeography and ecolo- gical phylogenetics in the three principal northern temperate genera of water striders Limnoporus STÅL 1868, Aquarius SCHELLENBERG 1800 and Gerris FABRICIUS 1794. Most of the discussion is based on com- parison of a recently published combined analysis tree involving four genetic markers and a morpholo- gical data set with older phylogenetic trees primarily based on manual cladistic optimization of mor- phological characters. Key words: DNA-barcodes, Gerrinae, phylogeography, simultaneous analyses. Introduction nally, water striders show great variation in mating strategies, and morphological and Water striders (Hemiptera-Heteroptera, behavioral adaptations to accomplish or Gerromorpha, Gerridae) are familiar inhab- avoid multiple mating (ANDERSEN 1994, itants of aquatic habitats throughout the 1996; ARNQVIST 1997). The striking diver- Worlds temperate, subtropical, and tropical sity in habitat selection, wing polymorphism regions comprising approximately 640 de- and mating strategies – along with the prac- scribed species in 72 genera (ANDERSEN & tically two dimensional habitat, has made WEIR 2004). Most water striders are found water striders popular objects in studies of in freshwater habitats, such as rivers, behavior, ecology and evolution (SPENCE & streams, lakes and ponds, but a few genera ANDERSEN 1994; ROWE et al. -

Nabs 2004 Final
CURRENT AND SELECTED BIBLIOGRAPHIES ON BENTHIC BIOLOGY 2004 Published August, 2005 North American Benthological Society 2 FOREWORD “Current and Selected Bibliographies on Benthic Biology” is published annu- ally for the members of the North American Benthological Society, and summarizes titles of articles published during the previous year. Pertinent titles prior to that year are also included if they have not been cited in previous reviews. I wish to thank each of the members of the NABS Literature Review Committee for providing bibliographic information for the 2004 NABS BIBLIOGRAPHY. I would also like to thank Elizabeth Wohlgemuth, INHS Librarian, and library assis- tants Anna FitzSimmons, Jessica Beverly, and Elizabeth Day, for their assistance in putting the 2004 bibliography together. Membership in the North American Benthological Society may be obtained by contacting Ms. Lucinda B. Johnson, Natural Resources Research Institute, Uni- versity of Minnesota, 5013 Miller Trunk Highway, Duluth, MN 55811. Phone: 218/720-4251. email:[email protected]. Dr. Donald W. Webb, Editor NABS Bibliography Illinois Natural History Survey Center for Biodiversity 607 East Peabody Drive Champaign, IL 61820 217/333-6846 e-mail: [email protected] 3 CONTENTS PERIPHYTON: Christine L. Weilhoefer, Environmental Science and Resources, Portland State University, Portland, O97207.................................5 ANNELIDA (Oligochaeta, etc.): Mark J. Wetzel, Center for Biodiversity, Illinois Natural History Survey, 607 East Peabody Drive, Champaign, IL 61820.................................................................................................................6 ANNELIDA (Hirudinea): Donald J. Klemm, Ecosystems Research Branch (MS-642), Ecological Exposure Research Division, National Exposure Re- search Laboratory, Office of Research & Development, U.S. Environmental Protection Agency, 26 W. Martin Luther King Dr., Cincinnati, OH 45268- 0001 and William E. -
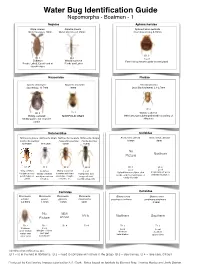
Water Bug ID Guide
Water Bug Identification Guide Nepomorpha - Boatmen - 1 Nepidae Aphelocheridae Nepa cinerea Ranatra linearis Aphelocheirus aestivalis Water Scorpion, 20mm Water Stick Insect, 35mm River Saucer bug, 8-10mm ID 1 ID 1 ID 1 Local Common Widely scattered Fast flowing streams under stones/gravel Ponds, Lakes, Canals and at Ponds and Lakes stream edges Naucoridae Pleidae Ilycoris cimicoides Naucoris maculata Plea minutissima Saucer bug, 13.5mm 10mm Least Backswimmer, 2.1-2.7mm ID 1 ID 1 ID 2 Widely scattered Widely scattered NORFOLK ONLY Often amongst submerged weed in a variety of Muddy ponds and stagnant stillwaters canals Notonectidae Corixidae Notonecta glauca Notonecta viridis Notonecta maculata Notonecta obliqua Arctocorisa gemari Arctocorisa carinata Common Backswimmer Peppered Backswimmer Pied Backswimmer 8.8mm 9mm 14-16mm 13-15mm 15mm 15mm No Northern Picture ID 2 ID 2 ID 2 ID 2 ID 3 ID 3 Local Local Very common Common Widely scattered Local Upland limestone lakes, dew In upland peat pools Ubiquitous in all Variety of waters In waters with hard Peat ponds, acid ponds, acid moorland lakes, or with little vegetation ponds, lakes or usually more base substrates, troughs, bog pools and sandy silt ponds canals rich sites concrete, etc. recently clay ponds Corixidae Corixidae Micronecta Micronecta Micronecta Micronecta Glaenocorisa Glaenocorisa scholtzi poweri griseola minutissima propinqua cavifrons propinqua propinqua 2-2.5mm 1.8mm 1.8mm 2mm 8.3mm No NEW RARE Northern Southern Picture ARIVAL ID 2 ID 2 ID 4 ID 4 ID 3 ID 3 Local Common Local Local Margins of rivers open shallow Northern Southern and quiet waters over upland lakes upland lakes silt or sand backwaters Identification difficulties are: ID 1 = id in the field in Northants. -

On the Benthic Water Bug Aphelocheirus Aestivalis (FABRICIUS 1794) (Heteroptera, Aphelocheiridae): Minireview 9-19 © Österr
ZOBODAT - www.zobodat.at Zoologisch-Botanische Datenbank/Zoological-Botanical Database Digitale Literatur/Digital Literature Zeitschrift/Journal: Entomologica Austriaca Jahr/Year: 2012 Band/Volume: 0019 Autor(en)/Author(s): Papacek Miroslav Artikel/Article: On the benthic water bug Aphelocheirus aestivalis (FABRICIUS 1794) (Heteroptera, Aphelocheiridae): Minireview 9-19 © Österr. Ent. Ges. [ÖEG]/Austria; download unter www.biologiezentrum.at Entomologica Austriaca 19 9-19 Linz, 16.3.2012 On the benthic water bug Aphelocheirus aestivalis (FABRICIUS 1794) (Heteroptera, Aphelocheiridae): Minireview M. PAPÁČEK Abstract: PAPÁČEK M.: On the benthic water bug Aphelocheirus aestivalis (FABRICIUS 1794) (Heteroptera, Aphelocheiridae): Minireview. Diagnostic characters of Aphelochirus aestivalis are listed, re-examined and figured in detail. Distribution, habitats, conservation status and biology of the species are briefly reviewed. Key words: Aphelocheirus aestivalis, diagnosis, distribution, habitats, biology. Introduction Aphelocheirus (Aphelocheirus) aestivalis (FABRICIUS 1794) was described as Naucoris aestivalis by FIEBER (1794: 66) who also characterized type material locality only by brief note: ‘Habitat in Galliae aquis Muf. Dom. Bofc.’. Historical name Gallia was used by Romans for Belgium, France, northern Italy, western Switzerland and parts of the Netherland and Germany. FABRICIUS (1794) meant most probably France. Exact holo- type locality is unknown and holotype is missing. For this reason LANSBURY (1965, p. 109) designated the lectotype (&, France) that is deposited in The Oxford University Museum, Hope Entomological Collections, Oxford, Great Britain. Furthermore KANYUKOVA (1995, p. 61) surveyed the synonymy of A. aestivalis (shortened version see below): Aphelocheirus breviceps HORVÁTH 1895: 160 (syn. KANYUKOVA 1974: 1730) Aphelocheirus kervillei KUHLGATZ 1898: 114 (syn. HORVÁTH 1899: 262) Aphelocheirus nigrita HORVÁTH 1899: 257, 263 (syn. -

A Review of the Hemiptera of Great Britain: the Aquatic and Semi-Aquatic Bugs
Natural England Commissioned Report NECR188 A review of the Hemiptera of Great Britain: The Aquatic and Semi-aquatic Bugs Dipsocoromorpha, Gerromorpha, Leptopodomorpha & Nepomorpha Species Status No.24 First published 20 November 2015 www.gov.uk/natural -england Foreword Natural England commission a range of reports from external contractors to provide evidence and advice to assist us in delivering our duties. The views in this report are those of the authors and do not necessarily represent those of Natural England. Background Making good decisions to conserve species should primarily be based upon an objective process of determining the degree of threat to the survival of a species. The recognised international approach to undertaking this is by assigning the species to one of the IUCN threat categories. This report was commissioned to update the national status of aquatic and semi-aquatic bugs using IUCN methodology for assessing threat. It covers all species of aquatic and semi-aquatic bugs (Heteroptera) in Great Britain, identifying those that are rare and/or under threat as well as non-threatened and non-native species. Reviews for other invertebrate groups will follow. Natural England Project Manager - Jon Webb, [email protected] Contractor - A.A. Cook (author) Keywords - invertebrates, red list, IUCN, status reviews, Heteroptera, aquatic bugs, shore bugs, IUCN threat categories, GB rarity status Further information This report can be downloaded from the Natural England website: www.gov.uk/government/organisations/natural-england. For information on Natural England publications contact the Natural England Enquiry Service on 0845 600 3078 or e-mail [email protected]. -

Ceh Code List for Recording the Macroinvertebrates in Fresh Water in the British Isles
01 OCTOBER 2011 CEH CODE LIST FOR RECORDING THE MACROINVERTEBRATES IN FRESH WATER IN THE BRITISH ISLES CYNTHIA DAVIES AND FRANÇOIS EDWARDS CEH Code List For Recording The Macroinvertebrates In Fresh Water In The British Isles October 2011 Report compiled by Cynthia Davies and François Edwards Centre for Ecology & Hydrology Maclean Building Benson Lane Crowmarsh Gifford, Wallingford Oxfordshire, OX10 8BB United Kingdom Purpose The purpose of this Coded List is to provide a standard set of names and identifying codes for freshwater macroinvertebrates in the British Isles. These codes are used in the CEH databases and by the water industry and academic and commercial organisations. It is intended that, by making the list as widely available as possible, the ease of data exchange throughout the aquatic science community can be improved. The list includes full listings of the aquatic invertebrates living in, or closely associated with, freshwaters in the British Isles. The list includes taxa that have historically been found in Britain but which have become extinct in recent times. Also included are names and codes for ‘artificial’ taxa (aggregates of taxa which are difficult to split) and for composite families used in calculation of certain water quality indices such as BMWP and AWIC scores. Current status The list has evolved from the checklist* produced originally by Peter Maitland (then of the Institute of Terrestrial Ecology) (Maitland, 1977) and subsequently revised by Mike Furse (Centre for Ecology & Hydrology), Ian McDonald (Thames Water Authority) and Bob Abel (Department of the Environment). That list was subject to regular revisions with financial support from the Environment Agency. -

Sovraccoperta Fauna Inglese Giusta, Page 1 @ Normalize
Comitato Scientifico per la Fauna d’Italia CHECKLIST AND DISTRIBUTION OF THE ITALIAN FAUNA FAUNA THE ITALIAN AND DISTRIBUTION OF CHECKLIST 10,000 terrestrial and inland water species and inland water 10,000 terrestrial CHECKLIST AND DISTRIBUTION OF THE ITALIAN FAUNA 10,000 terrestrial and inland water species ISBNISBN 88-89230-09-688-89230- 09- 6 Ministero dell’Ambiente 9 778888988889 230091230091 e della Tutela del Territorio e del Mare CH © Copyright 2006 - Comune di Verona ISSN 0392-0097 ISBN 88-89230-09-6 All rights reserved. No part of this publication may be reproduced, stored in a retrieval system, or transmitted in any form or by any means, without the prior permission in writing of the publishers and of the Authors. Direttore Responsabile Alessandra Aspes CHECKLIST AND DISTRIBUTION OF THE ITALIAN FAUNA 10,000 terrestrial and inland water species Memorie del Museo Civico di Storia Naturale di Verona - 2. Serie Sezione Scienze della Vita 17 - 2006 PROMOTING AGENCIES Italian Ministry for Environment and Territory and Sea, Nature Protection Directorate Civic Museum of Natural History of Verona Scientifi c Committee for the Fauna of Italy Calabria University, Department of Ecology EDITORIAL BOARD Aldo Cosentino Alessandro La Posta Augusto Vigna Taglianti Alessandra Aspes Leonardo Latella SCIENTIFIC BOARD Marco Bologna Pietro Brandmayr Eugenio Dupré Alessandro La Posta Leonardo Latella Alessandro Minelli Sandro Ruffo Fabio Stoch Augusto Vigna Taglianti Marzio Zapparoli EDITORS Sandro Ruffo Fabio Stoch DESIGN Riccardo Ricci LAYOUT Riccardo Ricci Zeno Guarienti EDITORIAL ASSISTANT Elisa Giacometti TRANSLATORS Maria Cristina Bruno (1-72, 239-307) Daniel Whitmore (73-238) VOLUME CITATION: Ruffo S., Stoch F.