Studies of Budding and Cell Wall Structure of Yeast
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cell Wall Chemistry Roger M
3 Cell Wall Chemistry Roger M. Rowell1,3, Roger Pettersen1, James S. Han1, Jeffrey S. Rowell2, and Mandla A. Tshabalala 1USDA, Forest Service, Forest Products Laboratory, Madison, WI 2Department of Forest Ecology and Management, University of Wisconsin, Madison, WI 3Department of Biological Systems Engineering, University of Wisconsin, Madison, WI CONTENTS 3.1 Carbohydrate Polymers ..........................................................................................................37 3.1.1 Holocellulose ..............................................................................................................37 3.1.2 Cellulose .....................................................................................................................37 3.1.3 Hemicelluloses............................................................................................................39 3.1.3.1 Hardwood Hemicelluloses ..........................................................................41 3.1.3.2 Softwood Hemicelluloses............................................................................42 3.1.4 Other Minor Polysaccharides .....................................................................................43 3.2 Lignin......................................................................................................................................43 3.3 Extractives ..............................................................................................................................45 3.4 Bark.........................................................................................................................................46 -

The Origin of Alternation of Generations in Land Plants
Theoriginof alternation of generations inlandplants: afocuson matrotrophy andhexose transport Linda K.E.Graham and LeeW .Wilcox Department of Botany,University of Wisconsin, 430Lincoln Drive, Madison,WI 53706, USA (lkgraham@facsta¡.wisc .edu ) Alifehistory involving alternation of two developmentally associated, multicellular generations (sporophyteand gametophyte) is anautapomorphy of embryophytes (bryophytes + vascularplants) . Microfossil dataindicate that Mid ^Late Ordovicianland plants possessed such alifecycle, and that the originof alternationof generationspreceded this date.Molecular phylogenetic data unambiguously relate charophyceangreen algae to the ancestryof monophyletic embryophytes, and identify bryophytes as early-divergentland plants. Comparison of reproduction in charophyceans and bryophytes suggests that the followingstages occurredduring evolutionary origin of embryophytic alternation of generations: (i) originof oogamy;(ii) retention ofeggsand zygotes on the parentalthallus; (iii) originof matrotrophy (regulatedtransfer ofnutritional and morphogenetic solutes fromparental cells tothe nextgeneration); (iv)origin of a multicellularsporophyte generation ;and(v) origin of non-£ agellate, walled spores. Oogamy,egg/zygoteretention andmatrotrophy characterize at least some moderncharophyceans, and arepostulated to represent pre-adaptativefeatures inherited byembryophytes from ancestral charophyceans.Matrotrophy is hypothesizedto have preceded originof the multicellularsporophytes of plants,and to represent acritical innovation.Molecular -

Cell Wall Ribosomes Nucleus Chloroplast Cytoplasm
Cell Wall Ribosomes Nucleus Nickname: Protector Nickname: Protein Maker Nickname: Brain The cell wall is the outer covering of a Plant cell. It is Ribosomes read the recipe from the The nucleus is the largest organelle in a cell. The a strong and stiff and made of DNA and use this recipe to make nucleus directs all activity in the cell. It also controls cellulose. It supports and protects the plant cell by proteins. The nucleus tells the the growth and reproduction of the cell. holding it upright. It ribosomes which proteins to make. In humans, the nucleus contains 46 chromosomes allows water, oxygen and carbon dioxide to pass in out They are found in both plant and which are the instructions for all the activities in your of plant cell. animal cells. In a cell they can be found cell and body. floating around in the cytoplasm or attached to the endoplasmic reticulum. Chloroplast Cytoplasm Endoplasmic Reticulum Nickname: Oven Nickname: Gel Nickname: Highway Chloroplasts are oval structures that that contain a green Cytoplasm is the gel like fluid inside a The endoplasmic reticulum (ER) is the transportation pigment called chlorophyll. This allows plants to make cell. The organelles are floating around in center for the cell. The ER is like the conveyor belt, you their own food through the process of photosynthesis. this fluid. would see at a supermarket, except instead of moving your groceries it moves proteins from one part of the cell Chloroplasts are necessary for photosynthesis, the food to another. The Endoplasmic Reticulum looks like a making process, to occur. -

The Structure, Function, and Biosynthesis of Plant Cell Wall Pectic Polysaccharides
Carbohydrate Research 344 (2009) 1879–1900 Contents lists available at ScienceDirect Carbohydrate Research journal homepage: www.elsevier.com/locate/carres The structure, function, and biosynthesis of plant cell wall pectic polysaccharides Kerry Hosmer Caffall a, Debra Mohnen a,b,* a University of Georgia, Department of Biochemistry and Molecular Biology and Complex Carbohydrate Research Center, 315 Riverbend Road Athens, GA 30602, United States b DOE BioEnergy Science Center (BESC), 315 Riverbend Road Athens, GA 30602, United States article info abstract Article history: Plant cell walls consist of carbohydrate, protein, and aromatic compounds and are essential to the proper Received 18 November 2008 growth and development of plants. The carbohydrate components make up 90% of the primary wall, Received in revised form 4 May 2009 and are critical to wall function. There is a diversity of polysaccharides that make up the wall and that Accepted 6 May 2009 are classified as one of three types: cellulose, hemicellulose, or pectin. The pectins, which are most abun- Available online 2 June 2009 dant in the plant primary cell walls and the middle lamellae, are a class of molecules defined by the pres- ence of galacturonic acid. The pectic polysaccharides include the galacturonans (homogalacturonan, Keywords: substituted galacturonans, and RG-II) and rhamnogalacturonan-I. Galacturonans have a backbone that Cell wall polysaccharides consists of -1,4-linked galacturonic acid. The identification of glycosyltransferases involved in pectin Galacturonan a Glycosyltransferases synthesis is essential to the study of cell wall function in plant growth and development and for maxi- Homogalacturonan mizing the value and use of plant polysaccharides in industry and human health. -

IAWA Bulletin N.S., Vol. 3 (1),1982 3 ANTONI V an LEEUWENHOEK
IAWA Bulletin n.s., Vol. 3 (1),1982 3 ANTONI VAN LEEUWENHOEK AND HIS OBSERVATION ON THE STRUCTURE OF THE WOODY CELL WALL by Pieter Baas Rijksherbarium, Leiden, The Netherlands Summary Following general remarks on the life of Van be traced back to negative but ill-informed Leeuwenhoek and his role in wood anatomy, judgements in some authoritative 19th and his account of the structure of a torn vessel 20th century publications (Baas, 1982a, b). wall of nutmeg rootwood is discussed in detail. Van Leeuwenhoek should be credited with The cross-wise orientation of minute 'vessels or many detailed and original wood and bark ana fibres' as observed and interpreted by Van tomical observations, but his work is not easily Leeuwenhoek can be considered to be the first accessible, being scattered in numerous letters, (unintentional) correct record of the fibrillar most of them also dealing with other microsco nature of the woody cell wall. pic subjects. Although most of these letters were published in various instalments and sev Introduction eral languages during Van Leeuwenhoek's life Antoni van Leeuwenhoek was born in 1632, time, this presentation cannot compete with 350 years ago, in Delft (the Netherlands) as the the balanced treatises by Grew and Malpighi, son of a basket maker. Apprenticed to a cloth who each published books solely dealing with merchant, Van Leeuwenhoek's career started as plant structure and function. a shopkeeper. Later he would serve as usher to Interest in Van Leeuwenhoek's plant anato the aldermen, chief warden and wine-gauger of mical work has recently also been revived by the City of Delft, and surveyor to the Court of the rediscovery of some of his sections among Holland. -

Primary and Secondary Plant Cell Walls: a Comparative Overview*
36 New Zealand Journal of Forestry Science 36(1) PRIMARY AND SECONDARY PLANT CELL WALLS: A COMPARATIVE OVERVIEW* PHILIP J. HARRIS School of Biological Sciences, The University of Auckland Private Bag 92019, Auckland, New Zealand [email protected] (Received for publication 23 November 2005; revision 3 February 2006) ABSTRACT Light and transmission electron microscopy are used in studying wall morphology and histochemical methods, including immunocytochemistry, can be used to locate specific compounds in walls. All plant cell walls contain a fibrillar phase of cellulose microfibrils and a matrix phase which contains a high proportion of non-cellulosic polysaccharides that vary in their chemical structures, depending on wall type and plant taxon. The non-cellulosic polysaccharide compositions of three common wall types — lignified secondary walls, non-lignified secondary walls, and non-lignified primary walls — exemplify this. The principles used in constructing the most recent models of non-lignified primary walls can be used in modelling lignified secondary walls. Keywords: primary cell walls; secondary cell walls; cell-wall models; non- cellulosic polysaccharides; transmission electron microscopy; light microscopy; chemistry; lignified walls; immuno- cytochemistry; histochemistry INTRODUCTION This review provides a brief comparative overview of primary and secondary cell walls of seed plants. A primary wall is defined as a wall that is deposited while the cell is still enlarging, whereas a secondary wall is deposited on the primary wall after cell expansion has stopped, and can be seen in sections as a structurally distinct layer (or layers) that is often very much thicker than the primary wall (Harris 2005a). At maturity, the different cell types can be grouped according to whether they have only a primary wall or both a primary and a secondary wall. -

Cell Structure and Function in the Bacteria and Archaea
4 Chapter Preview and Key Concepts 4.1 1.1 DiversityThe Beginnings among theof Microbiology Bacteria and Archaea 1.1. •The BacteriaThe are discovery classified of microorganismsinto several Cell Structure wasmajor dependent phyla. on observations made with 2. theThe microscope Archaea are currently classified into two 2. •major phyla.The emergence of experimental 4.2 Cellscience Shapes provided and Arrangements a means to test long held and Function beliefs and resolve controversies 3. Many bacterial cells have a rod, spherical, or 3. MicroInquiryspiral shape and1: Experimentation are organized into and a specific Scientificellular c arrangement. Inquiry in the Bacteria 4.31.2 AnMicroorganisms Overview to Bacterialand Disease and Transmission Archaeal 4.Cell • StructureEarly epidemiology studies suggested how diseases could be spread and 4. Bacterial and archaeal cells are organized at be controlled the cellular and molecular levels. 5. • Resistance to a disease can come and Archaea 4.4 External Cell Structures from exposure to and recovery from a mild 5.form Pili allowof (or cells a very to attach similar) to surfacesdisease or other cells. 1.3 The Classical Golden Age of Microbiology 6. Flagella provide motility. Our planet has always been in the “Age of Bacteria,” ever since the first 6. (1854-1914) 7. A glycocalyx protects against desiccation, fossils—bacteria of course—were entombed in rocks more than 3 billion 7. • The germ theory was based on the attaches cells to surfaces, and helps observations that different microorganisms years ago. On any possible, reasonable criterion, bacteria are—and always pathogens evade the immune system. have been—the dominant forms of life on Earth. -

Roles of the Plant Cell Wall in Powdery Mildew Disease
Roles of the plant cell wall in powdery mildew disease resistance in Arabidopsis thaliana: PMR5 (POWDERY MILDEW RESISTANT 5) affects the acetylation of cell wall pectin By Candice Cherk Lim A dissertation submitted in partial satisfaction of the requirements for the degree of Doctor of Philosophy in Plant Biology in the Graduate Division of the University of California, Berkeley Committee in charge: Professor Shauna Somerville, Chair Professor Patricia Zambryski Associate Professor Mary Wildermuth Professor James Berger Spring 2013 Abstract Roles of the plant cell wall in powdery mildew disease resistance in Arabidopsis thaliana: PMR5 (POWDERY MILDEW RESISTANT 5) affects the acetylation of cell wall pectin by Candice Cherk Lim Doctor of Philosophy in Plant Biology University of California, Berkeley Professor Shauna Somerville, Chair The pmr5 (powdery mildew resistant 5) mutant was found in a screen for genes involved in susceptibility to Golovinomyces cichoracearum, a biotrophic pathogen that infects Arabidopsis. PMR5 is a member of the TBL (TRICHOME BIREFRINGENCE LIKE) family, which is composed of 46 functionally uncharacterized plant-specific proteins. Initial characterization of this mutant showed that pmr5-mediated disease resistance acts independently of the salicylic acid, jasmonic acid, and ethylene signal transduction pathways, and that there are changes in the pmr5 cell wall that may be linked to the gain of resistance in the mutant. Specifically, PMR5 may be affecting cell wall pectin by acetylation. Characterization of the pmr5 cell wall has revealed changes in pectin composition and a decrease in acetylation. This is corroborated by the ability of heterologously expressed PMR5 protein to bind to pectin, with decreased binding affinity to acetylated pectin. -
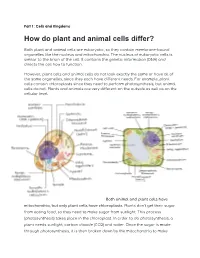
How Do Plant and Animal Cells Differ?
Part 1: Cells and Kingdoms How do plant and animal cells differ? Both plant and animal cells are eukaryotic, so they contain membrane-bound organelles like the nucleus and mitochondria. The nucleus of eukaryotic cells is similar to the brain of the cell. It contains the genetic information (DNA) and directs the cell how to function. However, plant cells and animal cells do not look exactly the same or have all of the same organelles, since they each have different needs. For example, plant cells contain chloroplasts since they need to perform photosynthesis, but animal cells do not. Plants and animals are very different on the outside as well as on the cellular level. Both animal and plant cells have mitochondria, but only plant cells have chloroplasts. Plants don’t get their sugar from eating food, so they need to make sugar from sunlight. This process (photosynthesis) takes place in the chloroplast. In order to do photosynthesis, a plant needs sunlight, carbon dioxide (CO2) and water. Once the sugar is made through photosynthesis, it is then broken down by the mitochondria to make energy for the cell. Because animals get sugar from the food they eat, they do not need chloroplasts: just mitochondria. Both plant and animal cells have vacuoles. A plant cell contains a large, singular vacuole that is used for storage of water and nutrients. It also helps maintain the shape of the cell. In contrast, animal cells have many, smaller vacuoles, which also are used for storage of water and nutrients. Plant cells have a cell wall, as well as a cell membrane. -

The Cell Wall of Green Microalgae and Its Role in Heavy Metal Removal
Received: 14 December 2020 Accepted: 15 March 2021 DOI: 10.1111/ppl.13405 SPECIAL ISSUE ARTICLE Physiologia Plantarum The cell wall of green microalgae and its role in heavy metal removal Olivia Spain | Martin Plöhn | Christiane Funk Department of Chemistry, Umeå University, Umeå Abstract Heavy metals in industrial wastewaters are posing a serious threat to the environ- Correspondence Christiane Funk, Department of Chemistry, ment and to human health. Microalgae are increasingly being seen as potential solu- Umeå University, 901 87 Umeå, Sweden. tions to this problem as they can remove pollutants through biosorption. This Email: [email protected] process offers certain advantages over other more traditional metal removal tech- Funding information niques as it is simple, inexpensive, eco-friendly, and can be performed over a wide Energimyndigheten, Grant/Award Number: 2018-017772; NordForsk, Grant/Award range of experimental conditions. Biosorption is possible due to the unique and com- Number: 82845; Svenska Forskningsrådet plex structure of the microalgal cell wall. The variety of functional groups on the sur- Formas, Grant/Award Number: 2019-00492; Umeå Universitet; Vinnova, Grant/Award face of the cell wall (such as carboxyl or amino groups) can act as binding sites for the Number: 2017-03301 heavy metals, thus removing them from the environment. This review focuses on the Edited by P.-E. Jensen cell wall composition and structure of the most commonly used microalgae in heavy metal removal and shows the role of their cell wall in the biosorption process. This review also aims to report the most commonly used models to predict the velocity of microalgal biosorption and the removal capacities. -

The Protists
The Protists (160,000 living & extinct species; estimates to 200,000 actual species) mostly single-celled, eukaryotes restricted to aquatic, or moist environments: oceans, ponds, lakes, rivers, damp soil, tree bark, snow, etc.; some species are colonial or multicellular; autotrophs & heterotrophs; with or without cell walls; most motile. Genetic analyses have dramatically changed the classification scheme if this group of organisms. In this course we will discuss examples of three main types of protists; algae, protozoa and slime & water molds. Algae [Plant-Like Protists] (22,000 living species) diverse group of mostly unicellular protists, some colonial or multicellular; restricted to aquatic or moist environments: oceans, ponds, lakes, rivers, soil, bark, snow, etc.; photosynthetic with chloroplasts containing chlorophyll and other pigments, most motile; most with cell wall; cell walls of cellulose, proteins,, silica or other materials classified according to their kinds of photosynthetic pigments and composition of cell wall Fire Algae (Dinoflagellates; Phylum Pyrrhophyta, 2100 species) unicellular, many symbiotic as zooxanthellae; some produce cell walls of armored plates, blooms produce toxic red tides, bioluminescent Diatoms (Glass Algae; Phylum Chrysophyta, 28000 living & extinct species) most abundant group of algae; unicellular, radial symmetry, cell walls contain silica; common in freshwater and oceans; source of diatomaceous earth; gliding movement, Euglenas (Phylum Euglenophyta, 1000 species) unicellular, only algae without -

4.3 Degradation of Plant Cell Walls and Membranes by Microbial Enzymes
4.3 Degradation of Plant Cell Walls and Membranes by Microbial Enzymes D.F. BATEMAN and H.G. BASHAM 1. Introduction One characteristic feature of many phytopathogenic organisms is their ability to produce an array of enzymes capable of degrading the complex polysaccharides of the plant cell wall (BATEMAN and MILLAR, 1966; WOOD, 1967; ALBERSHEIM et aI., 1969; WOOD, 1973) and membrane constituents (PORTER, 1966; TSENG and BATEMAN, 1968). These enzymes usually are produced inductively. Generally, they are extracellular, highly stable and present in infected host tissues. In most plant diseases caused by microbial agents, cell walls are penetrated, tissues are colonized, and permeability of host cells is altered. A brief summary of our understanding of cell wall and membrane structure, coupled with knowledge of the enzymes capable of degrading the components of these structures, and an analysis of the association of these enzymes with diseased tissue, should enable us to make an appraisal of their involvement in pathogenesis and point the way to an objective consideration of this area of disease physiology. 2. Structural Components 2.1 Cell Wall Composition and Structure The plant cell wall is that structure surrounding the protoplast exterior to the plasmalemma. This structure may be viewed as a two-phase system--- a dispersed phase of cellulose microfibrils and a complex continuous matrix. During develop ment cell walls undergo conspicious changes in structure, form and function, and they exhibit dynamic, rapid changes in their various constituents (NEVINS et aI., 1968; BERLYN, 1970; NORTHCOTE, 1972). Cell walls in young tissues are composed primarily of polysaccharides and a structural protein rich in hydroxyproline (LAM PORT, 1970).