The Bacterial Cell Wall
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Review Pili in Gram-Negative and Gram-Positive Bacteria – Structure
Cell. Mol. Life Sci. 66 (2009) 613 – 635 1420-682X/09/040613-23 Cellular and Molecular Life Sciences DOI 10.1007/s00018-008-8477-4 Birkhuser Verlag, Basel, 2008 Review Pili in Gram-negative and Gram-positive bacteria – structure, assembly and their role in disease T. Profta,c,* and E. N. Bakerb,c a School of Medical Sciences, Department of Molecular Medicine & Pathology, University of Auckland, Private Bag 92019, Auckland 1142 (New Zealand), Fax: +64-9-373-7492, e-mail: [email protected] b School of Biological Sciences, University of Auckland, Auckland (New Zealand) c Maurice Wilkins Centre for Molecular Biodiscovery, University of Auckland (New Zealand) Received 08 August 2008; received after revision 24 September 2008; accepted 01 October 2008 Online First 27 October 2008 Abstract. Many bacterial species possess long fila- special form of bacterial cell movement, known as mentous structures known as pili or fimbriae extend- twitching motility. In contrast, the more recently ing from their surfaces. Despite the diversity in pilus discovered pili in Gram-positive bacteria are formed structure and biogenesis, pili in Gram-negative bac- by covalent polymerization of pilin subunits in a teria are typically formed by non-covalent homopo- process that requires a dedicated sortase enzyme. lymerization of major pilus subunit proteins (pilins), Minor pilins are added to the fiber and play a major which generates the pilus shaft. Additional pilins may role in host cell colonization. be added to the fiber and often function as host cell This review gives an overview of the structure, adhesins. Some pili are also involved in biofilm assembly and function of the best-characterized pili formation, phage transduction, DNA uptake and a of both Gram-negative and Gram-positive bacteria. -
Prokaryotes (Domains Bacteria & Archaea)
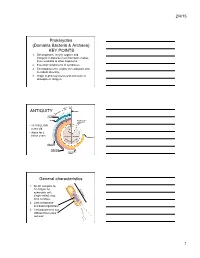
2/4/15 Prokaryotes (Domains Bacteria & Archaea) KEY POINTS 1. Decomposers: recycle organic and inorganic molecules in environment; makes them available to other organisms. 2. Essential components of symbioses. 3. Encompasses the origins of metabolism and metabolic diversity. 4. Origin of photosynthesis and formation of atmospheric Oxygen Ceno- Meso- zoic zoic ANTIQUITY Humans Paleozoic Colonization of land Animals Origin of solar system and Earth • >3.5 BILLION years old. • Alone for 2 1 4 billion years Proterozoic Archaean Prokaryotes Billions of 2 years ago3 Multicellular eukaryotes Single-celled eukaryotes Atmospheric oxygen General characteristics 1. Small: compare to 10-100µm for 0.5-5µm eukaryotic cell; single-celled; may form colonies. 2. Lack membrane- enclosed organelles. 3. Cell wall present, but different from plant cell wall. 1 2/4/15 General characteristics 4. Occur everywhere, most numerous organisms. – More individuals in a handful of soil then there are people that have ever lived. – By far more individuals in our gut than eukaryotic cells that are actually us. General characteristics 5. Metabolic diversity established nutritional modes of eukaryotes. General characteristics 6. Important decomposers and recyclers 2 2/4/15 General characteristics 6. Important decomposers and recyclers • Form the basis of global nutrient cycles. General characteristics 7. Symbionts!!!!!!! • Parasites • Pathogenic organisms. • About 1/2 of all human diseases are caused by Bacteria General characteristics 7. Symbionts!!!!!!! • Parasites • Pathogenic organisms. • Extremely important in agriculture as well. Pierce’s disease is caused by Xylella fastidiosa, a Gamma Proteobacteria. It causes over $56 million in damage annually in California. That’s with $34 million spent to control it! = $90 million in California alone. -

General Microbiology (11:680:390) Syllabus
COURSE SYLLABUS General Microbiology - 11:680:390 COURSE OVERVIEW General Microbiology 11:680:390 Fall, Spring, Summer Meeting times TBD Meeting Location Lecture: Sychronous Lecture Hall Cook/Douglas and Wright Labs Busch Meeting Location Lab: Food Science 209 CONTACT INFORMATION: Course Coordinator: Dr. Ines Rauschenbach Office Location: Lipman Hall, Room 215 Phone: 848-932-5635 Email: [email protected] Office Hours: By Appointment COURSE WEBSITE, RESOURCES AND MATERIALS: • Canvas • Text: Madigan MT, Bender KS, Buckley DH, Sattley WM, Stahl DA. 2020. Brock Biology of Microorganisms. 16th edition. Pearson, New York, NY. • Lab Manual o The lab manual (departmental publication) will be available for free through RUCore. • Electronic Notebook o We will be sending you a link to LabArchives. You must sign up before the start of your first lab. COURSE DESCRIPTION: This course offers a comprehensive study of the field of microbiology to science majors. The course will give detailed insights into five major themes: Structure and function of microbes (cellular structures, metabolism, and growth);,microbial genetics, microbial ecology, microbial diversity (prokaryotes, eukaryotes, viruses) and clinical microbiology (immunity, pathogenicity, epidemiology, control of microbes, and diseases). The course is taught in the synchronous lecture halls on Cook/Douglass and Busch campuses. Students are expected to participate in active learning activities and participate in class discussion to deepen their understanding of the microbial world and apply their knowledge to various concepts. LEARNING GOALS: Learning Goals for General Microbiology Lecture: After completion of the lecture component of the course, successful students will: 1. Demonstrate an understanding of the structural similarities and differences among microbes and the unique structure/function relationships of prokaryotic cells. -

A Generic Large-Scale Cause for Platelet Dysfunction and Depletion in Infection
Published online: 2020-04-12 THIEME 302 A Champion of Host Defense: A Generic Large-Scale Cause for Platelet Dysfunction and Depletion in Infection Martin J. Page, BSc (Hons)1 Etheresia Pretorius, PhD1 1 Department of Physiological Sciences, Stellenbosch University, Address for correspondence Etheresia Pretorius, PhD, Department of Stellenbosch, South Africa Physiological Sciences, Faculty of Science, Stellenbosch University, Private Bag X1 Matieland, Stellenbosch, 7602, South Africa Semin Thromb Hemost 2020;46:302–319. (e-mail: [email protected]). Abstract Thrombocytopenia is commonly associated with sepsis and infections, which in turn are characterized by a profound immune reaction to the invading pathogen. Platelets are one of the cellular entities that exert considerable immune, antibacterial, and antiviral actions, and are therefore active participants in the host response. Platelets are sensitive to surrounding inflammatory stimuli and contribute to the immune response by multiple mechanisms, including endowing the endothelium with a proinflammatory phenotype, enhancing and amplifying leukocyte recruitment and inflammation, promoting the effector functions of immune cells, and ensuring an optimal adaptive immune response. During infection, pathogens and their products influence the platelet response and can even be toxic. However, platelets are able to sense and engage bacteria and viruses to assist in their removal and destruction. Platelets greatly contribute to host defense by multiple mechanisms, including forming immune complexes and aggregates, shedding their granular content, and internalizing pathogens and subsequently being marked for removal. These processes, and the nature of platelet function in general, cause the platelet to be irreversibly consumed in Keywords the execution of its duty. An exaggerated systemic inflammatory response to infection ► platelets can drive platelet dysfunction, where platelets are inappropriately activated and face ► virus immunological destruction. -

The Capsule of Porphyromonas Gingivalis Reduces the Immune Response of Human Gingival Fibroblasts
UvA-DARE (Digital Academic Repository) The capsule of Porphyromonas gingivalis reduces the immune response of human gingival fibroblasts Brunner, J.; Scheres, N.; El Idrissi, N.B.; Deng, D.M.; Laine, M.L.; van Winkelhoff, A.J.; Crielaard, W. DOI 10.1186/1471-2180-10-5 Publication date 2010 Document Version Final published version Published in BMC Microbiology License CC BY Link to publication Citation for published version (APA): Brunner, J., Scheres, N., El Idrissi, N. B., Deng, D. M., Laine, M. L., van Winkelhoff, A. J., & Crielaard, W. (2010). The capsule of Porphyromonas gingivalis reduces the immune response of human gingival fibroblasts. BMC Microbiology, 10, [5]. https://doi.org/10.1186/1471-2180-10-5 General rights It is not permitted to download or to forward/distribute the text or part of it without the consent of the author(s) and/or copyright holder(s), other than for strictly personal, individual use, unless the work is under an open content license (like Creative Commons). Disclaimer/Complaints regulations If you believe that digital publication of certain material infringes any of your rights or (privacy) interests, please let the Library know, stating your reasons. In case of a legitimate complaint, the Library will make the material inaccessible and/or remove it from the website. Please Ask the Library: https://uba.uva.nl/en/contact, or a letter to: Library of the University of Amsterdam, Secretariat, Singel 425, 1012 WP Amsterdam, The Netherlands. You will be contacted as soon as possible. UvA-DARE is a service provided by the library of the University of Amsterdam (https://dare.uva.nl) Download date:29 Sep 2021 Brunner et al. -

G:\CLASSES\BI 345N6\Bi345n6 W07\Biol 345 W07
BIOLOGY 345 Name _____________________ Midterm I - 05 February 2007 PART I. Multiple choice questions – (4 points each, 36 points total). 1. Which of the following metals was used in the construction of pipes in early Rome and may have contributed to the fall of the Roman emprire? A. Iron B. Bronze C. Gold D. Lead E. Silver 2. Louis Pasteur is recognized as the scientist who finally refuted which hypothesis using experiments involving microorganisms and swan-necked flasks? A. Germ Theory B. Spontaneous generation C. Natural selection D. Ontogeny recapitulates phylogeny E. Pasteurization principle 3. Cell walls are important features in both bacteria and archaea. Which of the following componds best describes the biomolecular subunits one might find exclusively in an archaeal cell wall? A. Diaminopimelic acid (DAP) & D-alanine interbridge B. L-lysine & pentaglycine interbridge C. N-acetylglucosamine (NAG) & N-acetylmuramic acid (NAM) glycan D. N-acetylglucosamine (NAG) & N-acetyltalosaminuronic acid (NAT) glycan E. Dipicolinic acid & Ca++ 4. Considering the multitude of potential metabolic processes available to prokaryotes, which of the following are used to describe specific types of chemotrophic metabolisms? A. Energy source B. Carbon source C. Electron source D. Hydrogen source E. Electron acceptor Page 1 of 8 5. The majority of the bacterial cell’s dry weight (96.1% in E. coli) is due to just a few macromolecules and polymers. Which of the following is NOT a major component of a bacterial cell? A. RNA B. Peptidoglycan (aka murein) C. Proteins D. Vitamins E. Lipids 6. Which of the following is an invariant feature found among all microbial cells? A. -

Infection at the Wildlife-Livestock-Human Interface: Three Systems
Infection at the Wildlife- livestock-human interface: three systems Thesis submitted in accordance with the requirements of the University of Liverpool for the degree of Doctor in Philosophy by Elsa Sandoval Barron 12/4/2017 Abstract Zoonoses involve interactions between at least three species: the pathogen and two hosts, one of which is human and the other a non-human (vertebrate) animal. More than 60% of human infectious diseases are zoonotic, and many have a wildlife host. Urbanisation and human population growth have increased the demand for food and land resources, which have increased interaction between humans, domestic animals and wildlife and thus the potential for cross-species transmission of infections. Most studies of such systems take place in tropical and developing countries where population change and biodiversity makes the emergence of high profile infections (eg Ebola and SARS) more likely. This study, however, focuses on four well known infections within the UK: bovine tuberculosis, water-borne cryptosporidiosis and giardiasis, and campylobacteriosis. The aim of this study was to investigate, using four infectious diseases of economic and public health importance in the UK as study systems, the role of wildlife in the epidemiology of multihost, zoonotic infections. Bovine tuberculosis (bTB) is an important zoonosis in many parts of the world, but human infection is rare in the UK owing to a policy of ‘test and cull’ in cattle and pasteurisation of milk. However, there has been an epidemic of bTB in British cattle in recent decades, the control of which is complicated by infection in badgers (Meles meles) and controversy over the control of wildlife infection. -

Laboratory Exercises in Microbiology: Discovering the Unseen World Through Hands-On Investigation
City University of New York (CUNY) CUNY Academic Works Open Educational Resources Queensborough Community College 2016 Laboratory Exercises in Microbiology: Discovering the Unseen World Through Hands-On Investigation Joan Petersen CUNY Queensborough Community College Susan McLaughlin CUNY Queensborough Community College How does access to this work benefit ou?y Let us know! More information about this work at: https://academicworks.cuny.edu/qb_oers/16 Discover additional works at: https://academicworks.cuny.edu This work is made publicly available by the City University of New York (CUNY). Contact: [email protected] Laboratory Exercises in Microbiology: Discovering the Unseen World through Hands-On Investigation By Dr. Susan McLaughlin & Dr. Joan Petersen Queensborough Community College Laboratory Exercises in Microbiology: Discovering the Unseen World through Hands-On Investigation Table of Contents Preface………………………………………………………………………………………i Acknowledgments…………………………………………………………………………..ii Microbiology Lab Safety Instructions…………………………………………………...... iii Lab 1. Introduction to Microscopy and Diversity of Cell Types……………………......... 1 Lab 2. Introduction to Aseptic Techniques and Growth Media………………………...... 19 Lab 3. Preparation of Bacterial Smears and Introduction to Staining…………………...... 37 Lab 4. Acid fast and Endospore Staining……………………………………………......... 49 Lab 5. Metabolic Activities of Bacteria…………………………………………….…....... 59 Lab 6. Dichotomous Keys……………………………………………………………......... 77 Lab 7. The Effect of Physical Factors on Microbial Growth……………………………... 85 Lab 8. Chemical Control of Microbial Growth—Disinfectants and Antibiotics…………. 99 Lab 9. The Microbiology of Milk and Food………………………………………………. 111 Lab 10. The Eukaryotes………………………………………………………………........ 123 Lab 11. Clinical Microbiology I; Anaerobic pathogens; Vectors of Infectious Disease….. 141 Lab 12. Clinical Microbiology II—Immunology and the Biolog System………………… 153 Lab 13. Putting it all Together: Case Studies in Microbiology…………………………… 163 Appendix I. -

Some English Terms Used in Microbiology 1
Some English terms used in Microbiology 1 Shapes & Structures General terms Antibiotics and related Bacillus (pl. bacilli) Acid fast (acid fastness) ácido-alcohol resistente Acetylases Capsule Bacterial (adj.) Ampicillin Cell wall pared celular Bacterium (pl., bacteria): Beta-lactamase Coccus (cocci; and hence Staphylococcus, Bench: poyata Beta-lactamic Staphylococci) Biofilm Cephalosporin Core oligosaccharide núcleo oligosacarídico Burner (Bunsen Burner): mechero (Bunsen) Chloramphenicol Cortex Colony: colonia Colistin Fimbria (pl. fimbriae) Coverslip: (vidrio) cubreobjetos D-Cycloserine Flagellum (pl. flagella) Dye colorante DNA-gyrase Glycocalix Eukaryote or eucaryote Erythromycin Lipid A Incubator: estufa de incubación Ethambuthol Lipopolysaccharide Inoculating loop asa de siembra Fluoroquinolone Murein mureína Inoculum (inocula): Gentamicin (formerly gentamycin) Omp: outer membrane major protein proteína To flame: flamear Isoniazide de membrane externa Flask (Erlenmeyer flask): matraz Methicillin Outer membrane membrana externa Volumetric flask: matraz aforado Methylases PAMP (pathogen associated molecular pattern): Microorganism Nalidixic acid patrón molecular asociado a patógeno Motility movilidad Penicillin Peptidoglycan peptidoglucano Mycoplasm Penicillin binding protein (PBP) Periplasm periplasma Negative staining tinción negativa Phosphonomycin Periplasmic space espacio periplásmico Petri dish: placa de Petri Phosphorylases Permeability barrier barrera de permeabilidad Prokaryote or procaryote Polymyxin Pilus (pl. pili) Rack: -

MYCOBACTERIA.Pdf
MYCOBACTERIA Mycobacteria are widespread organisms, typically living in and food sources. Tuberculosis and the leprosy organisms are obligate parasites and are not found as free-living members of the genus. Mycobacteria are aerobic and nonmotile bacteria (except for the species Mycobacterium marinum, which has been shown to be motile within macrophages) that are characteristically acid fast. Mycobacteria have an outer membrane. They do not have capsules, and most do not form endospores. The distinguishing characteristic of all Mycobacterium species is that the cell wall is thicker than in many other bacteria, which is hydrophobic, waxy, and rich in mycolic acids/mycolates. The cell wall consists of the hydrophobic mycolate layer and a peptidoglycan layer held together by a polysaccharide, arabinogalactan. The cell wall makes a substantial contribution to the hardiness of this genus. The biosynthetic pathways of cell wall components are potential targets for new drugs for tuberculosis. Fig. 1 Mycobacterial cell wall: 1-outer lipids, 2-mycolic acid, 3-polysaccharides (arabinogalactan), 4-peptidoglycan, 5-plasma membrane, 6-lipoarabinomannan (LAM), 7- phosphatidylinositol mannoside, 8-cell wall skeleton. NONTUBERCULOUS MYCOBACTERIA – RUNYON CLASSIFICATION Runyon classification is a system of identifying mycobacteria on the basis of pigmentation and growth condition of the organisms. The Runyon classification of nontuberculous mycobacteria based on the rate of growth, production of yellow pigment and whether this pigment was produced in the dark or only after exposure to light. It was introduced by Ernest Runyon in 1959 (Fig. 111). On these bases, the nontuberculous mycobacteria are divided into four groups: Photochromogens (Group I) - produce nonpigmented colonies when grown in the dark and pigmented colonies only after exposure to light and reincubation (1M. -

The Regulation of Arsenic Metabolism in Rhizobium Sp. NT-26
The regulation of arsenic metabolism in Rhizobium sp. NT-26 Paula Corsini Madeira University College London Thesis submitted for the degree of Doctor of Philosophy 2016 I, Paula Corsini Madeira, confirm that the work presented in this thesis is my own. Where information has been derived from other sources, I confirm that this has been indicated in the thesis. Date: Signed: II Abstract Arsenic (As) is a toxic metalloid and a major contaminant in terrestrial and aquatic environments. The two soluble forms, arsenite (AsIII) and arsenate (AsV) are toxic to most organisms. A range of phylogenetically distant bacteria are able to oxidize AsIII to the less toxic form, arsenate AsV using the periplasmic arsenite oxidase (AioBA). The two-component signal transduction system AioS/AioR and the AsIII-binding periplasmic protein AioX are required for AsIII oxidation and are involved in the transcriptional regulation of the aioBA operon. Most AsIII oxidisers can also reduce AsV to AsIII via the As (Ars) resistance system. The focus of this work was to understand the regulation of genes involved in AsIII oxidation and As resistance together with those involved in phosphate metabolism in the facultative chemolithoautotrophic AsIII oxidiser NT-26 grown under different conditions. Gene expression was studied by quantitative PCR in cells grown heterotrophically with and without AsIII or AsV in late-log and stationary phases. qPCR was optimised and suitable reference genes were chosen. The expression of genes involved in phosphate transport, sensing As and the genes aioX, aioS, aioR (AsIII-sensing and regulation) and aioB, aioA (AsIII oxidation) and cytC (cytochrome c) were also analysed in NT-26 grown heterotrophically in the presence or absence of AsIII or AsV at different growth stages (i.e., late-log and stationary phases). -

Antonie Van Leeuwenhoek Journal of Microbiology
Antonie van Leeuwenhoek Journal of Microbiology Kroppenstedtia pulmonis sp. nov. and Kroppenstedtia sanguinis sp. nov., isolated from human patients --Manuscript Draft-- Manuscript Number: ANTO-D-15-00548R1 Full Title: Kroppenstedtia pulmonis sp. nov. and Kroppenstedtia sanguinis sp. nov., isolated from human patients Article Type: Original Article Keywords: Kroppenstedtia species, Kroppenstedtia pulmonis, Kroppenstedtia sanguinis, polyphasic taxonomy, 16S rRNA gene, thermoactinomycetes Corresponding Author: Melissa E Bell, MS Centers for Disease Control and Prevention Atlanta, Georgia UNITED STATES Corresponding Author Secondary Information: Corresponding Author's Institution: Centers for Disease Control and Prevention Corresponding Author's Secondary Institution: First Author: Melissa E Bell, MS First Author Secondary Information: Order of Authors: Melissa E Bell, MS Brent A. Lasker, PhD Hans-Peter Klenk, PhD Lesley Hoyles, PhD Catherine Spröer Peter Schumann June Brown Order of Authors Secondary Information: Funding Information: Abstract: Three human clinical strains (W9323T, X0209T and X0394) isolated from lung biopsy, blood and cerebral spinal fluid, respectively, were characterized using a polyphasic taxonomic approach. Comparative analysis of the 16S rRNA gene sequences showed the three strains belonged to two novel branches within the genus Kroppenstedtia: 16S rRNA gene sequence analysis of W9323T showed closest sequence similarity to Kroppenstedtia eburnea JFMB-ATE T (95.3 %), Kroppenstedtia guangzhouensis GD02T (94.7 %) and strain X0209T (94.6 %); sequence analysis of strain X0209T showed closest sequence similarity to K. eburnea JFMB-ATE T (96.4 %) and K. guangzhouensis GD02T (96.0 %). Strains X0209T and X0394 were 99.9 % similar to each other by 16S rRNA gene sequence analysis. The DNA-DNA relatedness was 94.6 %, confirming that X0209T and X0394 belong to the same species.