The Woolwich Arsenal and Acadian Mines Kenneth Pryke
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Explosives Recognition and Awareness in Court Security
10/29/2015 Explosives recognition and awareness in Court Security INTRODUCTION Course Objectives Familiarize court security personnel with explosives and their illicit uses Familiarize court security personnel with methods of prevention and detection of explosive devices in a court security setting. Familiarize court security personnel with methods of dealing with potential or actual explosive devices as first responders. 1 10/29/2015 The FBI Bomb Data Center reports that 70% of all terrorist incidents involve the use of incendiary agents and explosives. We know that there have been attacks carried out against government facilities in the U.S. including court buildings using explosives. This is why it is important for court security personnel to be familiar with explosives and their use. This course is not an “EOD”, “TACTICS”or “BOMB TECH”course. Theintentofthiscourseistoprovidebasic knowledge of the relationship between explosives and court security functions. Officers should always rely on their own training, department policy and common sense when dealing with explosives. HISTORY OF EXPLOSIVES 2 10/29/2015 Beginnings Gunpowder first appeared in China around the 1st century AD and was used for fireworks. The first evidence of use in weapons appeared in Europe around the 13th century when projectiles were propelled through tubes. The first high explosive, “fulminating gold” was first mentioned in writings by German alchemist Sebald Schwaertzer in 1585. Italian chemist Asconio Sobrero discovered nitroglycerin in 1846 but the compound was very unstable and difficult to work with. Alfred Nobel, Sweden Advances Swedish scientist Albert Nobel invents a method of stabilizing nitroglycerin in 1866 and patents dynamite in 1867. -

London Regional Group Programme Card
The SCI London Regional Group is currently the largest of all SCI regional groupings with c.1,000 members in Central and Greater London, the counties of Essex, Hertfordshire, Bedfordshire, Surrey, Middlesex, Kent and Sussex. The majority of whom are based in the region and are employed in the chemical, pharmaceutical and allied industries. There is also a good percentage of members in education and academia. The Group’s geographical remit includes more than 60 universities and colleges, the City of London, Westminster and Parliament, headquarters or London offices of some of the largest industrial companies in the world, and a plethora of science museums, galleries, societies and associations. The Group has a long and continuous history and was established shortly after the foundation of the Society itself in 1881. Its first Chairman was Sir Frederick Abel, the co-inventor of cordite. The London Group organises a regular and dynamic schedule of activities throughout the year in SCI London Regional Group SCI London keeping with its mission statement ‘utilising our capital city to publicise science and allow networking opportunities for all’. The broad-based programme of general interest and specialist events attracts a wide range of attendees - from students and families to science professionals and politicians. The SCI London Regional Group has a strong relationship with UCL’s Chemical & Physical Society, the students’ society of UCL’s Chemistry department (and the oldest UCL student society), and sponsors their lecture programme. Contact Details If you would like more information about the Group and its activities, or if you would like to get involved in the organisation of events, please contact [email protected] London Regional Group Join SCI Today! If you are not yet a member, you are missing a chance to network with people across the chemical Programme Card Spring 2016 and chemical-using industries. -

Chapter 7 Carbohydrates
Chapter 7 Carbohydrates Chapter Objectives: • Learn how to classify carbohydrates. • Learn how to recognize molecules with chiral centers and draw Fischer projections. • Learn how to classify the monosaccharides, and learn their chemical and physical properties. • Learn about the disaccharides and oligosaccharides. • Learn the major types of polysaccharides and their structural and biological features. Mr. Kevin A. Boudreaux Angelo State University CHEM 2353 Fundamentals of Organic Chemistry Organic and Biochemistry for Today (Seager & Slabaugh) www.angelo.edu/faculty/kboudrea Biochemistry • Biochemistry is the study of the chemistry of biomolecules and living organisms. • In organic chemistry, we organized our study of carbon-containing molecules by functional group (alcohol, alkene, ketone, carboxylic acid, etc.). • In the first five chapters, we will take a look at several groups of important biological molecules, many of which have more than one functional group: carbohydrates (Ch. 7), lipids (Ch. 8), proteins and enzymes (Ch. 9 and 10), and nucleic acids (Ch. 11). • We will also examine the synthesis of proteins (Ch. 11), nutrition (Ch. 12) and metabolism (Ch. 13, 14), and important body fluids (Ch. 15). 2 Classification of Carbohydrates 3 Carbohydrates and Biochemistry • Carbohydrates are compounds of tremendous biological importance: – they provide energy through oxidation – they supply carbon for the synthesis of cell components – they serve as a form of stored chemical energy – they form part of the structures of some cells and tissues • Carbohydrates, along with lipids, proteins, nucleic acids, and other compounds are known as biomolecules because they are closely associated with living organisms. 4 Carbohydrates • Carbohydrates, or saccharides (saccharo is Greek for “sugar”) are polyhydroxy aldehydes or ketones, or substances that yield such compounds on hydrolysis. -

Carbohydrates and Biochemistry
Chapter 7 Notes CHEM 2353 Fundamentals of Organic Chemistry ChapterChapter 77 CarbohydratesCarbohydrates Organic and Biochemistry for Today (4th ed.) Spencer L. Seager / Michael R. Slabaugh Mr. Kevin A. Boudreaux Angelo State University www.angelo.edu/faculty/kboudrea 1 Carbohydrates and Biochemistry • Carbohydrates are compounds of tremendous biological importance: – they provide energy through oxidation – they supply carbon for the synthesis of cell components – they serve as a form of stored chemical energy – they form part of the structures of some cells and tissues • Carbohydrates, along with lipids, proteins, nucleic acids, and other compounds are known as biomolecules because they are closely associated with living organisms. Biochemistry is the study of the chemistry of biomolecules and living organisms. 2 Chapter 7 Notes ClassificationClassification ofof CarbohydratesCarbohydrates 3 Carbohydrates • Carbohydrates are polyhydroxy aldehydes or ketones, or substances that yield such compounds on hydrolysis HO C HOHC HOHC HOHC CH2OH ribose The term “carbohydrate” comes from the fact that when you heat sugars, you get carbon and water. 4 Chapter 7 Notes Classes of Carbohydrates • Monosaccharides contain a single polyhydroxy aldehyde or ketone unit (saccharo is Greek for “sugar”) (e.g., glucose, fructose). • Disaccharides consist of two monosaccharide units linked together by a covalent bond (e.g., sucrose). • Oligosaccharides contain from 3 to 10 monosaccharide units (e.g., raffinose). 5 Classes of Carbohydrates • Polysaccharides contain very long chains of hundreds or thousands of monosaccharide units, which may be either in straight or branched chains (e.g., cellulose, glycogen, starch). 6 Chapter 7 Notes TheThe StereochemistryStereochemistry ofof CarbohydratesCarbohydrates 7 Stereoisomers • Glyceraldehyde, the simplest carbohydrate, exists in two isomeric forms that are mirror images of each other: CHO CHO HOC H HOH C CH2OH CH2OH L-glyceraldehyde D-glyceraldehyde • These forms are stereoisomers of each other. -

Werrett-Science of Destruction
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by UCL Discovery 1 The Science of Destruction: Terrorism and Technology in the Nineteenth Century Simon Werrett (Accepted by Oxford Handbooks Online (Oxford history of terrorism in the nineteenth century) November 2012, Oxford University Press) “Today, the importance of explosives as an instrument for carrying out revolutions oriented to social justice is obvious. Anyone can see that these materials will be the decisive factor in the next period of world history. It therefore makes sense for revolutionaries in all countries to acquire explosives and to learn the skills needed to use them in real situations.”1 The radical socialist publisher Johann Most, writing in his Science of Revolutionary Warfare in 1885, called his comrades’ attention to a wide range of explosive technologies developed by chemists working for the state and military in recent decades. The full title of his book, published after Most emigrated from Europe to the USA in 1882, listed some of these compositions, including “Nitroglycerine, Dynamite, Gun-Cotton, Fulminating Mercury, Bombs, [and] Fuses”, not to mention the more traditional knives, pistols, rifles, and poisons. Clockwork mechanisms were also used to explode ‘infernal machines’ of various kinds. He insisted, however, that the book was not what today would be called an ‘anarchist cookbook’. In his introduction, Most claimed that although many people had tried to produce these explosives in clandestine workshops, few had -

Chemical Wisdom- Horse Chestnuts and the Fermentation of Powerful Powders
Chemical Wisdom- Horse Chestnuts and the Fermentation of Powerful Powders Nitrocellulose, Guncotton, Cordite, Nitrogylcerine, Ballistite, Smokeless Powder, Acetone, Poudre B, Acetone-butanol-ethanol fermentation, Horse Chestnut. cellulose nitrate , flash paper , flash cotton , flash string is a highly flammable compound formed by nitrating cellulose through exposure to nitric acid or another powerful nitrating agent. When used as a propellant or low-order explosive , it was originally known as guncotton . Nitrocellulose plasticized by camphor was used by Kodak , and other suppliers, from the late 1880s as a film base in photograph, X-ray films and motion picture films; and was known as nitrate film . After numerous fires caused by unstable nitrate films, safety film started to be used from the 1930s in the case of X-ray stock and from 1948 for motion picture film. Guncotton Pure nitrocellulose Various types of smokeless powder, consisting primarily of nitrocellulose Henri Braconnot discovered in 1832 that nitric acid, when combined with starch or wood fibers, would produce a lightweight combustible explosive material, which he named xyloïdine . A few years later in 1838 another French chemist Théophile-Jules Pelouze (teacher of Ascanio Sobrero and Alfred Nobel ) treated paper and cardboard in the same way. He obtained a similar material he called nitramidine . Both of these substances were highly unstable, and were not practical explosives. However, around 1846 Christian Friedrich Schönbein , a German-Swiss chemist, discovered a more practical solution. As he was working in the kitchen of his home in Basel , he spilled a bottle of concentrated nitric acid on the kitchen table. He reached for the nearest cloth, a cotton apron, and wiped it up. -

Forensic Chemistry and Explosives MODULE No.34: Specific Approach to Scene of Explosion
Weblinks • http://inventors.about.com/od/estartinventions/a/explosives.htm • http://www.ch.ic.ac.uk/vchemlib/mim/bristol/tnt/tnt_text.htm • http://www.funtrivia.com/en/subtopics/The-**EXPLOSIVE**-Quiz-266249.html • http://chemsee.com/explosives-detection/resources/test-results/ • http://www.nij.gov/topics/law-enforcement/investigations/crime- scene/guides/explosion-bombing/Pages/process.aspx Suggested Readings q Forensic Science in Criminal Investigation & Trials by Dr. B. R. Sharma q Explosives by Samuel Delvin q Criminalistics: An Introduction to Forensic Science by Richard Saferstein FORENSIC SCIENCE PAPER No.5: Forensic Chemistry and Explosives MODULE No.34: Specific approach to scene of explosion q Practical Bomb Scene Investigation by James T.Thurman q Aspects of Explosives Detection by Maurice Marshall and Jimmie C.Oxley q Explosives by Rudolf Meyer, Josef Kohler and Axel Homburg FORENSIC SCIENCE PAPER No.5: Forensic Chemistry and Explosives MODULE No.34: Specific approach to scene of explosion Interesting Facts q In 1888, Albert Nobel invented a dense smokeless powder explosive called ballistite and in1889, Sir James Dewar and Sir Frederick Abel invented another smokeless gunpowder called cordite. q Cordite was made of nitroglycerin, guncotton, and a petroleum substance gelatinized by addition of acetone. q TNT is relatively unstable and can detonate when dropped or when a vehicle carrying it is hit by an IED or a bullet. q A detection/sniffer dog is trained to and works at using its senses specially sense of smell to detect substances such as explosives, illegal drugs, wildlife scat, blood etc. q The bomb disposal suit is capable of deflecting or stopping projectiles that may come from an exploded device. -

Polymers As Binders and Plasticizers – Historical Perspective
| 1 1 Polymers as Binders and Plasticizers – Historical Perspective Ever since the introduction of nitrocellulose (NC) as an explosive fill in the 1850s, polymers have contributed considerably to advancements in the technology of both propellants and explosives. In addition to the specific instance of the use of NC polymer in explosive fills, applications of polymers have been most extensive in binders and plasticizers. Over the years, with the maturity of composite propellant and polymer bonded explosive technology, diverse classes of polymers have been devel- oped for binder applications, in order to meet the dual objectives of insensitivity and high performance. This chapter focuses on the historical development of polymers for propellant and explosive formulations, particularly as binders and plasticizers. 1.1 Nitrocellulose Nitrocellulose (NC) (Figure 1.1), a nitrated carbohydrate, was the first polymer to be used in energetic material formulations, particularly in smokeless propellants. NC was discovered by Christian Friedrich Schonbein in Basel and Rudolf Chris- tian Bottger in Frankfurt-am-Main around 1845–1847 [1]. Henri Braconnot and Theophile Jules Pelouze had unknowingly prepared NC in 1833 and 1838, respectively, in France. They named these combustible materials CH2ONO2 O O ONO2 H H n H ONO2 Nitrocellulose Figure 1.1 Chemical structure of nitrocellulose. Energetic Polymers: Binders and Plasticizers for Enhancing Performance, First Edition. How Ghee Ang and Sreekumar Pisharath. r 2012 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim. Published 2012 by WILEY-VCH Verlag GmbH & Co. KGaA c01 6 December 2011; 14:32:32 2 | 1 Polymers as Binders and Plasticizers – Historical Perspective xyloidine and nitramidine. The announcements from Schonbein and Bottger in 1846 that NC had been prepared, meant that their names have since been associated with the discovery and utilization of NC. -

The 1998 Dexter Award Address
Bull. Hist. Chem. 24 (1999) 1 THE 1998 DEXTER AWARD ADDRESS "FROM AN INSTRUMENT OF WAR TO AN INSTRUMENT OF THE LABORATORY: THE AFFINITIES CERTAINLY DO NOT CHANGE" CHEMISTS AND THE DEVELOPMENT OF MUNITIONS, 1785-1885 Sr . Mpf, Unvrt I dpl pld nd hnrd t b th r d th prph f n f pbltn, tn rpnt f th xtr Ard. I ht dd tht I fr th "Cnè r r l pdr nn l ht tn b hn I nfrd (2"f ph rt. It prtnt tht I hnr tht I hd bn nntd, fr I rnz tht I hv rt b th f n f h ttn b h bn thn f "prdl n" rrdn th h h pld n prtnt rl n th trjtr f n tr f htr. I lft th fld fr bt fftn r rrh n th htr f htr. Ornll, I was t thr b th lfln frnd (nd hr ntrtd n th pt f h r fr hh h bt f th n n bjt vr dffrnt fr th h n—nltl htr nd th f fnt tr f htr (. Mr rprtn—bt th th n vr, rrh n th fld l ntxt f th rltn h bn ht nrth hp f rt t th dx I hv ht t tp npt f fxd nrl p tht hv d ntrt n ntprr rnh n t bt hd nt t rtllrph nd nrl trtd h hlrl ttn . M rnttn was tra- tn, t lt t th t I b ditional and "internalist" (to n td. -

Nitrocellulose from Gun-Cotton to Early Cinema by Deac Rossell Version: 6 June 2000
Exploding Teeth, Unbreakable Sheets and Continuous Casting: Nitrocellulose from Gun-cotton to Early Cinema by Deac Rossell version: 6 June 2000 [Originally a lecture given at the Congress of the Fédération International des Archivs du Film, June 2-7, 2000 during their symposium “And God Created Nitrate....”, at the British Film Institute, London. Then published (with revisions) in This Film Is Dangerous!, ed. Roger Smither and Carol Surowiec (Brussels, 2002: International Federation of Film Archives [FIAF]). Text version before FIAF editing for publication.] I: The Origins of Celluloid The origins of celluloid lie in the discovery of a method of nitrating cellulose fibres, or, in plain words, soaking cellulose fibres, usually taken from cotton or wood or one of their many products, in nitric acid and adding any one of a number of solvents. This process was first experimentally investigated in the 1830s and 1840s, and led directly to the formation of the field of organic chemistry, the combination of naturally-occurring materials with a solvent or catalyst to produce synthetic materials that do not appear in nature and which have a wide variety of different properties. Two pioneering French scientists conducted experiments in the 1830s which in themselves were only hesitant steps towards the formation of a practical discipline of organic compounds, but which bequeathed the names of their experimental materials to the later history of celluloid manufacture. Henri Bracconot combined nitric acid and potato starch into a material he called “xyloidine” in 1833, and Théophile-Jules Pelouzé used nitric acid and paper to make a material he called “pyroxyline” in 1838.1 The first scientist to generate a useful material by combining nitric acid and cellulose, in the form of cotton, was Christian Friedrich Schönbein, a professor at the University of Basle. -

JFI Presentation of the Franklin Medal to Major General James Douglas
IO4 PRESENTATIONOF FRANKLIN MEDAL. [J.F.I. PROGRAMME OF MEETING, MAY 2I, 1919. Presentation of The Franklin Medal to Major General James Douglas McLachlan, C.B., D.S.O., oll behalf of His Britannic Majesty's Government for Sir James Dewar, D.Sc., LL.D., F.R.S.; Jack- sonian Professor of Experimental Philosophy, University of Cambridge; Fullerian Professor of Chemistry, Royal Institution, London. Presentation of The Franklin Medal to Major General George Owen Squier, Ph.D., Chief Signal Officer, United States Army. Address : " Some Aspects of the Signal Corps in the World War." By Major General Squier. PRESENTATION OF THE FRANKLIN MEDAL TO SIR JAMES DEWAR AND MAJOR GENERAL GEORGE OWEN SQUIER. IN calling the meeting to order the President of the Institute announced that the business of the meeting would be the annual presentation of the Institute's highest award, The Franklin Medal, in recognition of distinguished scientific and technical achieve- ment, and recognized Dr. Harry F. Keller, who made the follow- ing statement relative to the work of Sir James Dewar : Mr. President: It; was at the May meeting of the Institute, 1914, that you presented the first impressions of the beautiful Franklin MedaI to two illustrious men of science. This precedent has since been followed, and so we meet here today for the fifth time to pay our tribute to a pair of savants who in the judgment of the Institute have done most to advance our knowledge of physical science. As chance would have it oi1 the present occasion the time could not have been chosen more happily ; for in these days of May our Nation is jubilantly celebrating the return of her heroic sons and exultantly looking forward to the early conclusion of a glor- ious peace, And I regard it as a very special and rare privilege, Mr. -
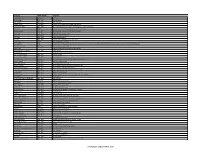
List of Famous Chemists in the World
Scientist Birth-Death Country Emil AbMderhalden 1877–1950 Swiss chemist Richard Abegg 1869–1910 German chemist Frederick Abel 1827–1902 English chemist Friedrich Accum 1769–1838 German chemist advances in the field of gas lighting Homer Burton Adkins 1892–1949 American chemist known for work in hydrogenation of organic compounds Peter Agre 1949– American chemist and doctor 2003 Nobel Prize in Chemistry Georgius Agricola 1494–1555 German scholar known as "the father of mineralogy"" Arthur Aikin 1773–1855 English chemist and mineralogist Adrien Albert 1907–1989 Australian medicinal chemist John Albery 1936–2013 English physical chemist Kurt Alder 1902–1958 German chemist 1950 Nobel Prize in Chemistry Sidney Altman 1939– 1989 Nobel Prize in Chemistry Faiza Al-Kharafi 1946– Kuwaiti chemist and academic. She was the president of Kuwait University from 1993 to 2002 and the first woman to head a major university in the Middle East. Christian B. Anfinsen 1916–1995 1972 Nobel Prize in Chemistry Angelo Angeli Brazilian Professor of Chemistry and Pharmaceutics Octavio Augusto Ceva Antunes 1731–1810 British scientist Anthony Joseph Joseph Arduengo III American chemist Johan August Arfwedson 1792–1841 Swedish chemist Anton Eduard van Arkel 1893–1976 Dutch chemist Svante Arrhenius 1859–1927 Swedish chemist one of the founders of physical chemistry Larned B. Asprey 1919–2005 American nuclear chemist Francis William Aston 1877–1945 1922 Nobel Prize in Chemistry Amedeo Avogadro 1776–1856 Italian chemist and physicist discovered Avogadro's law Stephen Moulton Babcock 1843–1931 worked on the "single-grain experiment" Werner Emmanuel Bachmann 1901–1951 American chemist known for work in steroids and RDX Leo Baekeland 1863–1944 Belgian-American chemist Adolf von Baeyer 1835–1917 German chemist 1905 Nobel Prize in Chemistry synthesis of indigo Piero Baglioni Italian chemist Hendrik Willem Bakhuis Roozeboom 1854–1907 Dutch chemist Allen J.