Acid/Base Basics How Does One Define Acids and Bases? in Chemistry, Acids and Bases Have Been Defined Differently by Three Sets of Theories
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
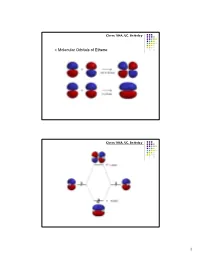
Π Molecular Orbitals of Ethene
Chem 104A, UC, Berkeley Molecular Orbitals of Ethene Chem 104A, UC, Berkeley 1 Chem 104A, UC, Berkeley Molecular Orbital Analysis of Ethene Dimerisation the reaction is said to be a "symmetry forbidden" – interestingly, this reaction is rare and very slow ! Chem 104A, UC, Berkeley Molecular Orbitals of 1,3-Butadiene 2 Chem 104A, UC, Berkeley Chem 104A, UC, Berkeley Molecular Orbital Analysis of Diels-Alder reaction 3 Chem 104A, UC, Berkeley Acid-Base Chemistry Reading : MT 6 Chem 104A, UC, Berkeley The Acid Base Theory of Brønsted and Lowry In 1923, within several months of each other, Johannes Nicolaus Brønsted (Denmark) and Thomas Martin Lowry (England) published essentially the same theory about how acids and bases behave. •An acid is a "proton donor." •A base is a "proton acceptor." 4 Chem 104A, UC, Berkeley + HCl + H2O <===> H3O + Cl¯ HCl - this is an acid, because it has a proton available to be transfered. H2O - this is a base, since it gets the proton that the acid lost. Now, here comes an interesting idea: + H3O - this is an acid, because it can give a proton. Cl¯ - this is a base, since it has the capacity to receive a proton. + Notice that each pair (HCl and Cl¯ as well as H2O and H3O differ by one proton (symbol = H+). These pairs are called conjugate pairs. Chem 104A, UC, Berkeley HCl + NaOH ---> H2O+ NaCl stronger stronger Weaker weaker conjugate conjugate acid base acid base + - NH4 +OH---> H2O+ NH3 stronger stronger weaker weaker conjugate conjugate acid base acid base 5 Chem 104A, UC, Berkeley H n A H 2O H n1 A H3O [H n1 A ][H3O ] pKa1 log [H n A] pKa 0 strong pKa 0 weak Chem 104A, UC, Berkeley Amphoteric Compound H n A H 2O H n1 A H3O NH 3 H 2O NH 4 OH 6 Chem 104A, UC, Berkeley Oxyacids AOp(OH)q A=Si, N, P, As, S, Se, Te, Cl, Br, I p=# of nonhydrogenated oxygen atom H + A ----O As atom A is rendered more positive, it becomes easier to break the O-H bond Because of enhanced bond polarization. -

A Guide to Acids, Acid Strength, and Concentration
A GUIDE TO ACIDS, ACID STRENGTH, AND CONCENTRATION What’s the difference between acid strength and concentration? And how does pH fit in with these? This graphic explains the basics. CH COOH HCl H2SO4 HNO3 H3PO4 HF 3 H2CO3 HYDROCHLORIC ACID SULFURIC ACID NITRIC ACID PHOSPHORIC ACID HYDROFLUORIC ACID ETHANOIC ACID CARBONIC ACID pKa = –7 pKa = –2 pKa = –2 pKa = 2.12 pKa = 3.45 pKa = 4.76 pKa = 6.37 STRONGER ACIDS WEAKER ACIDS STRONG ACIDS VS. WEAK ACIDS ACIDS, Ka AND pKa CONCENTRATION AND pH + – The H+ ion is transferred to a + A decrease of one on the pH scale represents + [H+] [A–] pH = –log10[H ] a tenfold increase in H+ concentration. HA H + A water molecule, forming H3O Ka = pKa = –log10[Ka] – [HA] – – + + A + + A– + A + A H + H H H H A H + H H H A Ka pK H – + – H a A H A A – + A– A + H A– H A– VERY STRONG ACID >0.1 <1 A– + H A + + + – H H A H A H H H + A – + – H A– A H A A– –3 FAIRLY STRONG ACID 10 –0.1 1–3 – – + A A + H – – + – H + H A A H A A A H H + A A– + H A– H H WEAK ACID 10–5–10–3 3–5 STRONG ACID WEAK ACID VERY WEAK ACID 10–15–10–5 5–15 CONCENTRATED ACID DILUTE ACID + – H Hydrogen ions A Negative ions H A Acid molecules EXTREMELY WEAK ACID <10–15 >15 H+ Hydrogen ions A– Negative ions Acids react with water when they are added to it, The acid dissociation constant, Ka, is a measure of the Concentration is distinct from strength. -

Properties of Acids and Bases
GREEN CHEMISTRY LABORATORY MANUAL Lab 22 Properties of Acids and Bases TN Standard 4.2: The student will investigate the characteristics of acids and bases. Have you ever brushed your teeth and then drank a glass of orange juice? hat do you taste when you brush your teeth and drink orange juice afterwards. Yuck! It leaves a really bad taste in your mouth, but why? Orange juice and toothpaste by themselves taste good. But the terrible taste W results because an acid/base reaction is going on in your mouth. Orange juice is a weak acid and the toothpaste is a weak base. When they are placed together they neutralize each other and produce a product that is unpleasant to taste. How do you determine what is an acid and what is a base? In this lab we will discover how to distinguish between acids and bases. Introduction Two very important classes of compounds are acids and bases. But what exactly makes them different? There are differences in definition, physical differences, and reaction differences. According to the Arrhenius definition, acids ionize in water to + produce a hydronium ion (H3O ), and bases dissociate in water to produce hydroxide ion (OH -). Physical differences can be detected by the senses, including taste and touch. Acids have a sour or tart taste and can produce a stinging sensation to broken skin. For example, if you have ever tasted a lemon, it can often result in a sour face. Bases have a bitter taste and a slippery feel. Soap and many cleaning products are bases. -

140. Sulphuric, Hydrochloric, Nitric and Phosphoric Acids
nr 2009;43(7) The Nordic Expert Group for Criteria Documentation of Health Risks from Chemicals 140. Sulphuric, hydrochloric, nitric and phosphoric acids Marianne van der Hagen Jill Järnberg arbete och hälsa | vetenskaplig skriftserie isbn 978-91-85971-14-5 issn 0346-7821 Arbete och Hälsa Arbete och Hälsa (Work and Health) is a scientific report series published by Occupational and Enviromental Medicine at Sahlgrenska Academy, University of Gothenburg. The series publishes scientific original work, review articles, criteria documents and dissertations. All articles are peer-reviewed. Arbete och Hälsa has a broad target group and welcomes articles in different areas. Instructions and templates for manuscript editing are available at http://www.amm.se/aoh Summaries in Swedish and English as well as the complete original texts from 1997 are also available online. Arbete och Hälsa Editorial Board: Editor-in-chief: Kjell Torén Tor Aasen, Bergen Kristina Alexanderson, Stockholm Co-editors: Maria Albin, Ewa Wigaeus Berit Bakke, Oslo Tornqvist, Marianne Törner, Wijnand Lars Barregård, Göteborg Eduard, Lotta Dellve och Roger Persson Jens Peter Bonde, Köpenhamn Managing editor: Cina Holmer Jörgen Eklund, Linköping Mats Eklöf, Göteborg © University of Gothenburg & authors 2009 Mats Hagberg, Göteborg Kari Heldal, Oslo Arbete och Hälsa, University of Gothenburg Kristina Jakobsson, Lund SE 405 30 Gothenburg, Sweden Malin Josephson, Uppsala Bengt Järvholm, Umeå ISBN 978-91-85971-14-5 Anette Kærgaard, Herning ISSN 0346–7821 Ann Kryger, Köpenhamn http://www.amm.se/aoh -

Hydrochloric Acid Handbook
Hydrochloric Acid Handbook OxyChem ® OxyChem is a registered trademark of Occidental Chemical Corp. 08/2018 Dallas-based Occidental Chemical Corporation is a leading North American manufacturer of basic chemicals, vinyls and performance chemicals directly and through various affiliates (collectively, OxyChem). OxyChem is also North America's largest producer of sodium chlorite. As a Responsible Care® company, OxyChem's global commitment to safety and the environment goes well beyond compliance. OxyChem's Health, Environment and Safety philosophy is a positive motivational force for our employees, and helps create a strong culture for protecting human health and the environment. Our risk management programs and methods have been, and continue to be, recognized as some of the industry's best. OxyChem offers an effective combination of industry expertise, experience, on line business tools, quality products and exceptional customer service. As a member of the Occidental Petroleum Corporation family, OxyChem represents a rich history of experience, top-notch business acumen, and sound, ethical business practices. Table of Contents Page INTRODUCTION TO HYDROCHLORIC ACID .................................................................................... 4 MANUFACTURING ........................................................................................................................... 4 HYDROCHLORIC ACID — USES ........................................................................................................ 5 SPECIFICATIONS AND -

Notes Acids and Bases
Notes Acids and Bases Arrhenius Acid Base Theory: In 1884 Svante Arrhenius proposed the first theoretical model for acids and bases. Prior to that time, these chemically opposite substances were described in properties such as their taste; their effects on metals, carbonates, and dyes (called indicators); their feel to the touch, and their ability to react with each other. According to the Arrhenius theory , pure water dissociates to some extent to produce hydrogen ions, H + and hydroxide ions, OH -. When this occurs, equal amounts of H + and OH - ions are produced: + - H2O(l) H (aq) + OH (aq) An acid, according to Arrhenius, is any substance that liberates H + ions when placed in water. When the H + concentration is elevated the OH - concentration decreases, this solution is said to be acidic. Similarly, a base is defined as any substance that liberates OH - ions when placed in water. The resulting solution has a higher concentration of OH - ions than H + ions and is said to be basic, or alkaline. + - ACID: HCl (aq) H (aq) + Cl (aq) + - BASE: NaOH (aq) Na (aq) + OH (aq) Brønsted - Lowry Acid Base Theory: A more general and realistic theory was proposed by two chemists working independent of the other, Johannes Nicolaus Brønsted of Denmark and Thomas Martin Lowry of England. Both chemists are given credit; the theory is named the Brønsted-Lowry theory . Acids are defined as substances that donate H + ions, protons and therefore are called proton donators, while bases are substances that accept protons and are defined as proton acceptors. On this basis, the autoionization of water is given as follows: + - H2O(l) + H 2O(l) H3O (aq) + OH (aq) In this reaction, one water molecule donates a proton, H +, and behaves as an acid, while the other water molecule accepts the proton and behaves as a base. -

Hydrochloric Acid MSDS Effective Date: December 03, 2012 24 Hour Emergency Contact: Chemtel: (800)255-3924
Hydrochloric Acid MSDS Effective Date: December 03, 2012 24 Hour Emergency Contact: ChemTel: (800)255-3924 www.pioneerforensics.com 1. PRODUCT AND COMPANY IDENTIFICATION Product: Hydrochloric Acid Product Number(s): PF021, PF022 CAS#: 7647-01-0 Synonyms: Muriatic acid; Hydrogen chloride, aqueous; Chlorohydric acid Manufacturer: Pioneer Forensics, LLC 804 E. Eisenhauer Blvd. Loveland, CO 80537 Ph: (970) 292-8487 Emergency Number: (800) 255-3924 (CHEM-TEL) Customer Service: (970) 292-8487 2. HAZARDS IDENTIFICATION Emergency Overview: DANGER! Corrosive. Causes severe skin, eye, and digestive tract burns. Harmful if swallowed. Mist or vapor extremely irritating to eyes and respiratory tract. Safety Ratings: Health: 3, Severe Reactivity: 1, Slight Flammability: 0, None Contact: 4, Extreme OSHA Regulatory Status: This product is considered a "Hazardous Chemical" as defined by the OSHA Hazard Communication Standard, 29 CFR 1910.1200. Potential Acute Health Effects: Routes of Exposure: Inhalation, ingestion, skin contact, eye contact Inhalation: Corrosive. May cause damage to mucous membranes in nose, throat, lungs and bronchial system. Ingestion: Corrosive. Harmful if swallowed. May produce burns to the lips, oral cavity, upper airway, esophagus and digestive tract. Skin Contact: Corrosive. Causes severe burns. Eye Contact: Corrosive. Causes severe burns. Vapor or spray may cause eye damage, impaired sight or blindness. Target Organs: Skin, respiratory system, eyes, lungs Chronic Health Effects: Corrosive. Prolonged contact causes serious tissue damage. Product: Hydrochloric Acid Revision Date: 12/03/2012 1/7 Aggravation of: Repeated or prolonged exposure to the substance can produce target organs damage. Medical Conditions: Persons with pre-existing skin disorders or eye problems may be more susceptible to the effects of the substance. -

The Activity and Other Thermodynamic Properties of Hydrochloric Acid in Tetrahydrofuran - Water Mixtures
Louisiana State University LSU Digital Commons LSU Historical Dissertations and Theses Graduate School 1966 The Activity and Other Thermodynamic Properties of Hydrochloric Acid in Tetrahydrofuran - Water Mixtures. Rabindra Nath Roy Louisiana State University and Agricultural & Mechanical College Follow this and additional works at: https://digitalcommons.lsu.edu/gradschool_disstheses Recommended Citation Roy, Rabindra Nath, "The Activity and Other Thermodynamic Properties of Hydrochloric Acid in Tetrahydrofuran - Water Mixtures." (1966). LSU Historical Dissertations and Theses. 1217. https://digitalcommons.lsu.edu/gradschool_disstheses/1217 This Dissertation is brought to you for free and open access by the Graduate School at LSU Digital Commons. It has been accepted for inclusion in LSU Historical Dissertations and Theses by an authorized administrator of LSU Digital Commons. For more information, please contact [email protected]. This dissertation has been microfilmed exactly as received 67-1184 ROY, Rabindra Nath, 1939- THE ACTIVITY AND OTHER THERMODYNAMIC PROPERTIES OF HYDROCHLORIC ACID IN TETRAHYDROFURAN-WATER MIXTURES. Louisiana State University and Agricultural and Mechanical College, Ph.D., 1966 Chemistry, physical University Microfilms, Inc., Ann Arbor, Michigan THE ACTIVITY AND OTHER THERMODYNAMIC PROPERTIES OF HYDROCHLORIC ACID IN TETRAHYDROFURAN-WATER MIXTURES A Dissertation Submitted to the Graduate Faculty of the Louisiana State University and Agricultural and Mechanical College in partial fulfillment of the requirements for the degree of Doctor of Philosophy in The Department of Chemistry by Rabindra Nath Roy B. Sc. (Hons); Jadavpur University, 1959 M. Sc.; Jadavpur University, 1961 August, 1966 ACKNOWLEDGMENT The author wishes to thank first his parents who long ago instilled in him a high appreciation for education. The author is grateful to Dr. -

Efficient Surface Functionalization of Detonation Nanodiamond Using
Electronic Supplementary Material (ESI) for Nanoscale. This journal is © The Royal Society of Chemistry 2019 Efficient surface functionalization of detonation nanodiamond using ozone under ambient conditions Johannes Ackermann,a Anke Kruegera,b* a Institute for Organic Chemistry, Julius-Maximilians University Würzburg, Am Hubland, D-97074 Würzburg, Germany, b Wilhelm Conrad Röntgen Center for Complex Materials Systems (RCCM), Julius-Maximilians University Würzburg, Am Hubland, D-97074 Würzburg, Germany *[email protected] Supporting information Raman spectra: Fig. S1 Raman spectrum of DND S1 Fig. S2 Raman spectrum of DNDox Fig. S3 Raman spectrum of DNDred S2 Fig. S4 Raman spectrum of aDND Fig. S5 Raman spectrum of aDNDox S3 Fig. S6 Raman spectrum of aDNDred XRD spectra: Fig. S7 XRD spectra of a) untreated DND b) DND treated with ozone and oxidized with H2O2 c) DND treated with ozone and reduced with NaBH4 S4 Fig. S8 XRD spectra of a) untreated aDND b) aDND treated with ozone and oxidized with H2O2 c) aDND treated with ozone and reduced with NaBH4 FTIR spectra of diamond materials between 1500 and 1800 cm-1 Fig. S9. Magnified FTIR spectra of a) untreated DND b) DND treated with ozone over a period of six hours c) DND treated with ozone for 6 h and oxidation with hydrogen peroxide d) DND treated with ozone for 6 h, oxidation with hydrogen peroxide and washing with dilute hydrochloric acid e) DND treated with ozone for 6 h, reduction with sodium borohydride and washing with dilute hydrochloric acid. S5 Fig. S10. FT-IR spectra of a) untreated aDND b) aDND treated with ozone over a period of six hours c) aDND treated with ozone for 6 h, oxidation with hydrogen peroxide and washing with dilute hydrochloric acid d) aDND treated with ozone for 6 h, reduction with sodium borohydride and washing with dilute hydrochloric acid. -

Ammonia, NH3 Is a Base. It Reacts with Hydrochloric Acid According to the Following Equation
Titration notes Name ___________________________________________ Warm up: How many moles of ammonia are in 75 milliliters of a 0.75M solution? How many moles of hydrochloric acid are in 25.0 mL of a 0.500 M solution? Acid base reaction Ammonia, NH3 is a base. It reacts with hydrochloric acid according to the following equation. Calculate the concentration of 25 mL of an HCl solution if it requires 45.5 mL of 0.75M NH3 solution to neutralize it. NH3 (aq) + HCl (aq) → H2O (l) + NH4Cl (aq) Titration What is the purpose of titration? What is the relationship between titration and neutralization? What is the equivalence point? Titration practice Log on to your text – you will not be going to your text but rather will be using the resources! Follow these steps: 1. Select the “menu” hamburger in the top left 2. Choose “resources” 3. Select “chapter 18” and “section 4: Neutralization” from the drop down menus 4. Find and choose the “animation: Neutralization” 5. You are now ready to begin. Start with the HCl/NaOH titration and answer the following before pressing any more buttons. 1. What is the balanced chemical equation for the reaction between hydrochloric acid and sodium hydroxide? 2. Which chemical is the titrant? 3. What is the concentration of the HCl? _________ What volume is in the flask? __________ 4. How many moles of HCl are in the flask? (show your work) 5. What is the concentration of the NaOH? __________________ 6. How many moles of NaOH will you need to neutralize the HCl? 7. -

Hydrochloric Acid
TECHNICAL BULLETIN 19 Motivation Dve Wangara, WA, 6065 AUSTRALIA T +61 8 9302 4000 | FREE 1800 999 196 | F +61 8 9302 5000 HYDROCHLORIC ACID A NON-OXIDIZING ACID MATERIAL & FUNCTION HYDROCHLORIC ACID is a clear, colourless, fuming, poisonous, highly acidic aqueous solution of hydrogen chloride (chemical symbol HCl). It is used as a chemical intermediate and in petroleum production, ore reduction, food processing, TECHNICAL BULLETIN 19 Motivation Dve Wangara, WA, 6065 AUSTRALIA T +61 8 9302 4000 | FREE 1800 999 196 | F +61 8 9302 5000 pickling, and metal cleaning. It is found in the stomach in dilute form. Synonyms: muriatic acid; chlorohydric acid; hydrochloride; spirits of salts Chemical properties: HYDROCHLORIC ACID is one of the most corrosive of the non-oxidizing acids in contact with copper alloys and is handled in dilute solutions. Contact with metals produces hydrogen gas which creates the chance of an explosion. It produces poisonous gas, including chlorine, in a fire. It is soluble in benzene, alcohol and ether It is insoluble in hydrocarbons and incompatible or reactive with metals, hydroxides, amines and alkalis. HYDROCHLORIC ACID’S fumes have an acid, penetrating odour. Aqueous solutions of HYDROCHLORIC ACID attack and corrode nearly all metals, except mercury, silver, gold, platinum, tantalum, and certain alloys. It may be coloured yellow by traces of iron, chlorine and organic matter. The physical properties of HYDROCHLORIC ACID, such as boiling and melting points, density and pH depend on the concentration or molarity of HCl in the acid solution. They can range from those of water at 0% HCl to values for fuming HYDROCHLORIC ACID at over 40% HCl. -

Incompatible Chemical Groups.Pdf
Incompatible Chemical Hazard Groups (and some common examples) Mineral Acids Do NOT Store with… Hydrochloric acid Hydrogen peroxide Acetone Sulfuric Acid Sodium hydroxide Methanol Phosphoric Acid Calcium hydroxide Nitric Acid (keep separate) Chloroform Acetic Acid Strong Organic Acids Do NOT Store with… Acetic Acid3, 4 Hydrogen peroxide Acetone Acetonitrile Formic Acid Sodium hydroxide Methanol Benzene Sulfuric Acid Chloroform Special 1. Organic acids are varied and may be incompatible with each other. Notes: Check MSDSs for specifics 2. Store nitric acid separately in its own secondary container. It is a strong oxidizer. 3. Store acetic acid away from oxidizing agents — especially nitric acid. 4. Acetic acid may be stored with some inorganic acids and most flammable solvents but keep in a separate secondary container. (>70% acetic acid is combustible). Weak These are typically not corrosive and not strongly reactive and can be Organic Acids stored with general liquid lab chemicals. Examples include butyric, maleic, and benzoic acids. Non-Flammable Do NOT Store with… Chlorinated Solvents Methylene chloride Acetone Hexane Chloroform Methanol Nitric Acid Trichloroethane Ethanol Hydrogen Peroxide Carbon tetrachloride Organic Solvents Do NOT Store with… Acetone Hydrogen peroxide Nitric Acid Methanol Sodium hydroxide Chromic Acid Phenol Calcium hydroxide Sulfuric Acid Xylene Trichlorfluoromethane Hydrochloric Acid Oxidizers Do NOT Store with… Nitric Acid Sodium metal Paper and oily rags Hydrogen peroxide Isopropyl Alcohol Xylene Chromic Acid Acetone Sodium nitrate Perchloric Acid Ethyl ether Bromate salts .