Carbone Monoxide (CO) Detection Device Based on the Nickel Antimonate Oxide and a DC Electronic Circuit
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

ON Semiconductor Is an Equal Opportunity/Affirmative Action Employer
ON Semiconductor Is Now To learn more about onsemi™, please visit our website at www.onsemi.com onsemi and and other names, marks, and brands are registered and/or common law trademarks of Semiconductor Components Industries, LLC dba “onsemi” or its affiliates and/or subsidiaries in the United States and/or other countries. onsemi owns the rights to a number of patents, trademarks, copyrights, trade secrets, and other intellectual property. A listing of onsemi product/patent coverage may be accessed at www.onsemi.com/site/pdf/Patent-Marking.pdf. onsemi reserves the right to make changes at any time to any products or information herein, without notice. The information herein is provided “as-is” and onsemi makes no warranty, representation or guarantee regarding the accuracy of the information, product features, availability, functionality, or suitability of its products for any particular purpose, nor does onsemi assume any liability arising out of the application or use of any product or circuit, and specifically disclaims any and all liability, including without limitation special, consequential or incidental damages. Buyer is responsible for its products and applications using onsemi products, including compliance with all laws, regulations and safety requirements or standards, regardless of any support or applications information provided by onsemi. “Typical” parameters which may be provided in onsemi data sheets and/ or specifications can and do vary in different applications and actual performance may vary over time. All operating parameters, including “Typicals” must be validated for each customer application by customer’s technical experts. onsemi does not convey any license under any of its intellectual property rights nor the rights of others. -

Chapter 12 Three-Phase Controlled Rectifiers ∫
CHAPTER 12 THREE-PHASE CONTROLLED RECTIFIERS Author : Juan Dixon (Ph.D.) Department of Electrical Engineering Pontificia Universidad Católica de Chile Vicuña Mackenna 4860 Santiago, CHILE. 12.1 INTRODUCTION Three-phase controlled rectifiers have a wide range of applications, from small rectifiers to large High Voltage Direct Current (HVDC) transmission systems. They are used for electro-chemical process, many kinds of motor drives, traction equipment, controlled power supplies, and many other applications. From the point of view of the commutation process, they can be classified in two important categories: Line Commutated Controlled Rectifiers (Thyristor Rectifiers), and Force Commutated PWM Rectifiers. 12.2 LINE COMMUTATED CONTROLLED RECTIFIERS. 12.2.1 Three- phase hal - wavef rectifie r The figure 12.1 shows the three-phase half-wave rectifier topology. To control the load voltage, the half wave rectifier uses three, common-cathode thyristor arrangement. In this figure, the power supply, and the transformer are assumed ideal. The thyristor will conduct (ON state), when the anode-to-cathode voltage vAK is positive, and a firing current pulse iG is applied to the gate terminal. Delaying the firing pulse by an angle a does the control of the load voltage. The firing angle a is measured from the crossing point between the phase supply voltages, as shown in figure 12.2. At that point, the anode-to-cathode thyristor voltage vAK begins to be positive. The figure 12.3 shows that the possible range for gating delay is between a=0° and a=180°, but in real situations, because of commutation problems, the maximum firing angle is limited to around 160°. -

Bremner, Duncan James (2015) 25 Years of Network Access Technologies: from Voice to Internet; the Changing Face of Telecommunications
Bremner, Duncan James (2015) 25 years of network access technologies: from voice to internet; the changing face of telecommunications. PhD thesis. http://theses.gla.ac.uk/6670/ Copyright and moral rights for this thesis are retained by the author A copy can be downloaded for personal non-commercial research or study, without prior permission or charge This thesis cannot be reproduced or quoted extensively from without first obtaining permission in writing from the Author The content must not be changed in any way or sold commercially in any format or medium without the formal permission of the Author When referring to this work, full bibliographic details including the author, title, awarding institution and date of the thesis must be given. Glasgow Theses Service http://theses.gla.ac.uk/ [email protected] 25 years of Network Access Technologies: From Voice to Internet; the changing face of telecommunications Duncan James Bremner A Thesis submitted to School of Engineering College of Science and Engineering University of Glasgow in fulfilment of the requirements for the Degree of Doctor of Philosophy by published work May 2015 Abstract This work contributes to knowledge in the field of semiconductor system architectures, circuit design and implementation, and communications protocols. The work starts by describing the challenges of interfacing legacy analogue subscriber loops to an electronic circuit contained within the Central Office (Telephone Exchange) building. It then moves on to describe the globalisation of the telecom network, the demand for software programmable devices to enable system customisation cost effectively, and the creation of circuit and system blocks to realise this. -

Users Guide for the Non-Inverted LM3875 Kit Written by Brian Bell and Sandy H
Users Guide for the Non-Inverted LM3875 Kit Written by Brian Bell and Sandy H. 1 (26) Users Guide for the Non-Inverted LM3875 Kit (also known as the Gainclone kit) 1 INTRODUCTION.......................................................................................................................................... 2 1.1 HISTORY ................................................................................................................................................... 3 1.2 TERMINOLOGY USED IN THIS DOCUMENT................................................................................................. 4 2 BUILDING INSTRUCTIONS FOR THE KIT ........................................................................................... 5 2.1 PREMIUM KIT CONTENTS ......................................................................................................................... 5 2.2 BASIC KIT CONTENTS............................................................................................................................... 5 2.3 SOLDERING TIPS ....................................................................................................................................... 6 2.4 ASSEMBLING THE AMPLIFIER PCB........................................................................................................... 7 2.5 ASSEMBLING THE RECTIFIER PCB ......................................................................................................... 13 2.6 PICTURE OF FINISHED PCBS .................................................................................................................. -
4 S1-Us A-Bo-Le Gist'y
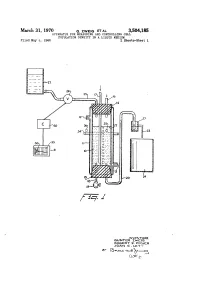
March 31, 1970 G. ZWEG ET All 3,504,185 APPARATUS FOR MEASURING AND CONTROLING CEL POPULATION DENSITY IN A LIQUID MEDIUM Filed May 9, 1968 2. Sheets-Sheet :9 O - 32. 33 : o SAHH 3. ?m WeavroAs, GUNT.R Z. We G ROBERT e. PP-ER JOAn . - 4 S1-us a-bo-le Gist'y. March 31, 1970 G. ZWEG ET All- 3,504,185 APPARATUS FOR MEASURING AND CONTROLLING CELL POPULATION DENSITY IN A LIQUID MEDIUM Filed May 9, 1968 2. Sheets-Sheet 2 N /Ayyam AOes. GUnTER ZWEG RO2ERT E. PPHER JOAN. E. HTT 3,504,185 United States Patent Office Patented Mar. 31, 1970 2 3,504,185 medium to dilute the cultured medium until the cultured APPARATUS FOR MEASURING AND CONTROL medium becomes less turbid again and the output of the LING CELL POPULATION DENSITY IN A LIQUID comparator is no longer energized so that the switch is MEDUM no longer operated and the valve is shut off. Gunter Zweig, Syracuse, Robert E. Pipher, Cortland, and A constant fluid level is maintained in the chamber Joan E. Hitt, Syracuse, N.Y., assignors to Syracuse Uni versity Research Corporation, Syracuse, N.Y., a corpo in which the cultured medium is contained and the over ration of New York flow resulting from dilution is collected for use. Usually Filed May 9, 1968, Ser. No. 727,910 continual agitation of the fluid in the chamber is required nt. C. G01n 21/26 to keep the cells in suspension in the medium and physical U.S. C. 250- 218 S Claims 10 conditions which are necessary for optimum growth speed are maintained in the usual manner. -

Lecture 8 - Effect of Source Inductance on Rectifier Operation
ELEC4240/9240 Power Electronics Lecture 8 - Effect of source inductance on rectifier operation Ideal VS real rectifier with source inductance The output DC voltages of the rectifier circuits discussed so far have been found by assuming that diode currents transfer (commutate) from one diode to another instantaneously. However this can not happen when the AC source has some inductance Ls. (Change of current through any inductance must take some time!). This source inductance is associated with the leakage inductance of the supply transformer and the inductance of the AC supply network to the input transformer. The commutation process (or the overlap process) forces more than one diode or a pair of diodes (in a bridge rectifier) to conduct simultaneously, resulting in a drop voltage from the output terminals which is proportional to the load current. The output dc voltage Vd of a rectifier falls with load current Id, by an amount which is much larger than additional voltage drop across the conducting diodes when the current through the diodes increases. The AC source inductance, which consists of the AC line and the input transformer leakage inductances, is mostly responsible for the additional voltage drop. Consider the half-wave diode rectifier shown below. D is Ls vs Id IDf D Load vs = Vmaxsinωt ∼ vi f Figure 8.1. Half-wave diode rectifier with source inductance. Let us assume that the load current Id is smooth and ripple-free (i.e., of constant, due to the highly inductive load). Assume also that for ωt > 0, the load current flows through the rectifier diode and that for ωt > π, it commutates to the free-wheeling diode Df. -

Project Administration Data Sheet
(7, ■■••••••"'". GEORGIA INSTITUTE OF TECHNOLOGY OFFICE OF CONTRACT ADMINISTRATION PROJECT ADMINISTRATION DATA SHEET ORIGINAL E REVISION NO. Project No. E-21-616 R58480A0 GTRIAW DATE 10/ 24/ 84 Project Director: Dr. J. A. Connelly SchooRgt EE Spimisort Honeywell Inc., Underseas System Division 600 Second Street Northeast Hopkins, MN 55343 Type Agreement: Unnumbered Research Agreement Award Period: From 9,'15/84 To (Performance) 12/15/84 (Reports) Sponsor Amount: This Change Total to Date Estima•:ed: $ 24,495 $ 24,495 Fundk: $ 24,495 $24,495 Cost Sharing Amount $ N/A Cost Sharing No: Title: Sea Water Bulk Sensor Design ADMINISTRATIVE DATA OCA Contact Dennis Farmer x41370 1) Sponsor Technical Contact: . 2) Sponsor Admin/Contractual Matters: X/ Curt Motchenbacher, Sr. Staff Engineer LaNeal Pewewardy, Subcontract Man ager Honeywell Inc._. Subsystems Procurement Underseas System Operations Honeywell Inc. 600 2nd Street 600 2nd Street N.E. Hopkins, MN 55343 Hopkins, MN 55343 (612) 931-6511 Defense Priority Rating: Military Security Classification: (or) Company/Industrial Proprietary: Non-;;.Di-scIosUreagre'emenil RESTRICTIONS See Attached Supplemental Information Sheet for Additional Requirements. Travel: Foreign travel must have prior approval — Contact OCA in each case. Domestic travel requires sponsor approval where tstal will exceed greater of $500 or 125% of approved proposal budget category none proposed Equipment: Title vests with 064 (.1 \ COMMENTS:. P.O. No. 130000 is referenced in the a Mr. Pewewar the P.O. because he thought the research agreement should be sufficient. should reference P.O. No. 130000 when submitted for payment. COPIES TO: - Project Director Procurement/E ES Supply Services GTRI Research Administrative Network Services Library . -

THD Mitigation in Line Currents of 6-Pulse Diode Bridge Rectifier Using the Delta-Wye Transformer As a Triplen Harmonic Filter A
16th NATIONAL POWER SYSTEMS CONFERENCE, 15th-17th DECEMBER, 2010 210 THD Mitigation in Line Currents of 6-Pulse Diode Bridge Rectifier Using the Delta-Wye Transformer as a Triplen Harmonic Filter A. N. Arvindan and B. Abinaya Dept. of Electrical & Electronics Engineering, SSN College of Engineering, Anna University (Chennai), India Abstract—The uncontrolled three-phase bridge rectifier has the least line current distortion among six-pulse rectifiers; however, its total harmonic distortion (THD) of 31.08% is unacceptable as per the contemporary power quality standards. This paper seeks to address the issue by elimination of triplen harmonics in the line currents by providing a delta- wye transformer at the ac interface i.e. between the three-phase supply and the diode bridge rectifier. The triplen harmonics circulate in the delta loop of the primary winding but are not manifested in the line currents thus reducing their THD values. The efficacy of the technique is proved by simulations considering the bridge rectifier with and without the delta-wye transformer of vector group Dy1. Experimental results with the Dy1 transformer and without it are presented. Keywords— diode bridge rectifier, improved power quality, total harmonic distortion, triplen harmonics. Fig. 1. An ac utility directly connected to a three-phase diode bridge rectifier feeding an inductive load. I. INTRODUCTION as stipulated by IEEE 519-1992. This paper investigates the HE three-phase diode bridge is, perhaps, the most effect of connecting the six-pulse diode bridge topology to T popular of the six-pulse rectifiers and is extensively the three-phase ac utility via a delta-wye (Δ-Y) transformer used, however, with the stringent contemporary power in terms of trapping the triplen harmonics in the delta loop, quality standards [1], [2] stipulating specific permissible thus eliminating them from the ac utility line currents and levels of total harmonic distortion (THD) at the ac utility also thereby improving the THD at the utility. -

Pulse Service Documentation
Crown Pulse Series Service Documenation The information furnished in this manual does not include all of the details of design, production, or variations of the equipment. Nor does it cover every possible situation which may arise during installation, operation or maintenance. If you need special assistance beyond the scope of this manual, please contact the Crown Technical Support Group. 1718 W. Mishawaka Road Elkhart IN 46517 Phone: (800) 342-6939 / (219) 294-8200 FAX: (219) 294-8301 CAUTION AVIS TO PREVENT ELECTRIC SHOCK DO NOT REMOVE TOP OR BOTTOM À PRÉVENIR LE CHOC COVERS. NO USER SERVICEABLE ÉLECTRIQUE N’ENLEVEZ PARTS INSIDE. REFER SERVICING PAS LES COUVERTURES. TO QUALIFIED SERVICE RIEN DES PARTIES PERSONNEL. DISCONNECT UTILES À L’INTÉRIEUR. POWER CORD BEFORE REMOVING DÉBRANCHER LA BORNE REAR INPUT MODULE TO ACCESS AVANT D’OUVRIR LA GAIN SWITCH. MODULE EN ARRIÈRE. WARNING TO REDUCE THE RISK OF ELECTRIC SHOCK, DO NOT EXPOSE THIS EQUIPMENT TO RAIN OR MOISTURE! The lightning bolt The exclamation point triangle is used to triangle is used to alert the alert the user to the user to important operating risk of electric shock. or maintenance instructions. ©2005 Crown Audio, Inc. Cautions and Warnings Lightning Bolt Symbol: Exclamation Mark Symbol: This symbol is used to alert the user This symbol is used to alert the user to to the presence of dangerous make special note of important voltages and the possible risk of operating or maintenance instructions. electric shock. DANGER: The outputs of the amplifier can produce LETHAL energy levels! Be very careful when making connections. Do not attempt to change output wiring until the amplifier has been off at least 10 seconds. -

Service Manual
Service Manual Issue 1.0 P1 AmpliÞ er AARCAMRCAM BBringingringing mmusicusic & mmoviesovies ttoo llifeife Contents List Circuit description Circuit diagrams o L929AY switch PCB o L962AY amplifier main PCB Transformers o L929TX – 115/230VAC toroidal o L927TX – 100VAC toroidal o L907TX – 100VAC frame Exploded view diagram Mechanical & packing parts list Circuit board silk screen & parts list o L929AY switch PCB o L962AY amplifier main PCB Fmj P1 Amplifier circuit description by A.Moore Power supply/Control description Product description The mains input is applied via SKT1. Y capacitors C1 and C2 and X capacitor, C5 The P1 has been has been designed to provide filtering and EMC suppression R2 provide unsurpassed sound quality, the main provides a discharge path for the capacitors. design features are as follows. SW1 allows the selection of the mains voltage that the unit will operate from, the main standby o Gain switchable between Arcam gain transformer TX1 and the main transformer TX2 and THX gain (29dB closed loop) a 0dB have duel primary windings, these windings are signal equates to 100 watts into 8 connected in parallel for 115v operation and in ohms. series for 230v operation. o Input switchable between unbalance TX1 is powered at all times when a mains phono and balance XLR. voltage is applied to the mains input socket the o The amp is capable of producing >180 secondary of this transformer is fused by via FS3 watts of sinusoidal output into an 8 and rectified by diode bridge DBR1 and ohm load and greater than 300 watts regulated to 5V by low dropout regulator at into a 3.2 ohm load (subject to thermal location REG2 to provide a constant +5v(D) dissipation limits). -

Introduction to Power Electronics
ELG3336: Power Electronics Systems Objective To Realize and Design Various Power Supplies and Motor Drives! Power electronics refers to control and conversion of electrical power by power semiconductor devices wherein these devices operate as switches. Advent of silicon-controlled rectifiers, abbreviated as SCRs, led to the development of a new field of application called the power electronics. Before SCRs, mercury-arc rectifiers were used for controlling electrical power, but such rectifier circuits were part of industrial electronics and the scope for applications of mercury-arc rectifiers was limited. The application spread to many fields such as drives, power supplies, aviation electronics, high frequency inverters and power electronics. 1 Why Power Electronics? Power electronics is a growing field due to the improvement in switching technologies and the need for more and more efficient switching circuits. Control Analog/Digital Electronics Power Equipment Devices/Circuits Static/Rotating 3 Applications • Heating and lighting control • Induction heating • Uninterruptible power supplies (UPS) • Fluorescent lamp ballasts: Passive; Active • Electric power transmission • Automotive electronics • Electronic ignitions • Motor drives • Battery chargers • Alternators • Energy storage • Electric vehicles • Alternative power sources: Solar; Wind; Fuel Cells • And more! 4 Power Train of a Hybrid Car Some Applications of Power Electronics • In a conventional car, power electronics applications are a major area of future expansion. Look inside the audio system, for example; the amplifiers in today’s car stereos are usually capable of delivering 40 W or more. But a 12 V supply applied to an 8 Ohm speaker produces 18 W output at best. To solve this power supply problem, designers use a boost converter (DC to DC Converter) to provide higher voltage power to the amplifier circuit. -

High-Voltage Direct-Current Transmission Converters, Systems and Dc Grids
HIGH-VOLTAGE DIRECT-CURRENT TRANSMISSION HIGH-VOLTAGE DIRECT-CURRENT TRANSMISSION CONVERTERS, SYSTEMS AND DC GRIDS Dragan Jovcic and Khaled Ahmed School of Engineering, University of Aberdeen, UK This edition first published 2015 © 2015 John Wiley & Sons, Ltd Registered Office John Wiley & Sons, Ltd, The Atrium, Southern Gate, Chichester, West Sussex, PO19 8SQ, United Kingdom For details of our global editorial offices, for customer services and for information about how to apply for permission to reuse the copyright material in this book please see our website at www.wiley.com. The right of the author to be identified as the author of this work has been asserted in accordance with the Copyright, Designs and Patents Act 1988. All rights reserved. No part of this publication may be reproduced, stored in a retrieval system, or transmitted, in any form or by any means, electronic, mechanical, photocopying, recording or otherwise, except as permitted by the UK Copyright, Designs and Patents Act 1988, without the prior permission of the publisher. Wiley also publishes its books in a variety of electronic formats. Some content that appears in print may not be available in electronic books. Designations used by companies to distinguish their products are often claimed as trademarks. All brand names and product names used in this book are trade names, service marks, trademarks or registered trademarks of their respective owners. The publisher is not associated with any product or vendor mentioned in this book. Limit of Liability/Disclaimer of Warranty: While the publisher and author have used their best efforts in preparing this book, they make no representations or warranties with respect to the accuracy or completeness of the contents of this book and specifically disclaim any implied warranties of merchantability or fitness for a particular purpose.