Material and Method
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Maharashtra Tourism Development Corporation Ltd., Mumbai 400 021
WEL-COME TO THE INFORMATION OF MAHARASHTRA TOURISM DEVELOPMENT CORPORATION LIMITED, MUMBAI 400 021 UNDER CENTRAL GOVERNMENT’S RIGHT TO INFORMATION ACT 2005 Right to information Act 2005-Section 4 (a) & (b) Name of the Public Authority : Maharashtra Tourism Development Corporation (MTDC) INDEX Section 4 (a) : MTDC maintains an independent website (www.maharashtratourism. gov.in) which already exhibits its important features, activities & Tourism Incentive Scheme 2000. A separate link is proposed to be given for the various information required under the Act. Section 4 (b) : The information proposed to be published under the Act i) The particulars of organization, functions & objectives. (Annexure I) (A & B) ii) The powers & duties of its officers. (Annexure II) iii) The procedure followed in the decision making process, channels of supervision & Accountability (Annexure III) iv) Norms set for discharge of functions (N-A) v) Service Regulations. (Annexure IV) vi) Documents held – Tourism Incentive Scheme 2000. (Available on MTDC website) & Bed & Breakfast Scheme, Annual Report for 1997-98. (Annexure V-A to C) vii) While formulating the State Tourism Policy, the Association of Hotels, Restaurants, Tour Operators, etc. and its members are consulted. Note enclosed. (Annexure VI) viii) A note on constituting the Board of Directors of MTDC enclosed ( Annexure VII). ix) Directory of officers enclosed. (Annexure VIII) x) Monthly Remuneration of its employees (Annexure IX) xi) Budget allocation to MTDC, with plans & proposed expenditure. (Annexure X) xii) No programmes for subsidy exists in MTDC. xiii) List of Recipients of concessions under TIS 2000. (Annexure X-A) and Bed & Breakfast Scheme. (Annexure XI-B) xiv) Details of information available. -

ANJANERI PLATEAU, Nashik District ______Anjaneri Plateau Is One of the Important Hill Fort Anjaneri Area Disturbance in the Mountain Range of Nasik- (Sq
ANJANERI PLATEAU, Nashik District ____________________________________________________ Anjaneri plateau is one of the important hill fort Anjaneri Area Disturbance in the mountain range of Nasik- (sq. Tryambakeshwar. It is located 20 km away kms) from Nasik by Tryambak Road. The rocky hills Plateau 1 local grazing, fires, of Tryambak (famous Jyotirling), Brahmagiri Plateau 2 6.3821 trampling and and Anjaneri are well known sacred places Plateau 3 1.6491 wasteful picking by and part of religious pilgrimage circuit for Total 8.0312 tourists, devotees. The plateau top can be reached plant collection Illegal extraction for after a steep climb from Anjaneri village. It is sale. believed to be the birthplace of Hanuman, son of Anjani, and a temple dedicated to Anjani Mata is built on the plateau top. The mesa has steep cliff edges which descend into gently sloping hill slopes. The plateau and The hill top is an exposed basalt plateau its surrounding steep slopes have forest located between 19°53'39.12"N, patches affected by biotic pressures. Dense 73°34'48.20"E to 19°56'19.02"N, forest is seen only in less accessible areas. 73°34'28.56"E. The highest point is around 1300 MSL. The fort has 3 extensive plateaus With an exception of a few hectares land at the elevation of 800MSL, 1100 MSL and under private ownership, the entire area is 1280-1300 MSL respectively under RF category. Forest of the fort is divided into four Gram-Panchayats namely, Anjaneri, funding and local support by the Territorial Mulegaon, Pegalwadi and Pahine. There are Forest Department of Nashik circle. -

5 Easy Weekend Getaways from Mumbai.Indd 170 31/12/19 4:06 PM TRAVEL GETAWAYS
TRAVEL GETAWAYS MURUD-JANJIRA 5 EASY WEEKEND Western Maharashtra is blessed with a wonderful coastline stretching 720 km and dotted with beaches – both popular and GETAWAYS pristine. Beach bums can capitalise on the weekend and go beach hopping along Maharashtra’s coastline. Of all the beaches FROM MUMBAI along the coastline, Murud Janjira is a must visit. TAKE A BREAK FROM MUMBAI’S HUSTLE AND BUSTLE TO HIT THESE WEEKEND The Janjira Fort, ruled by the Siddis of GETAWAYS. DISCOVER PLACES FOR HISTORY LOVERS, NATURE ENTHUSIASTS, WINE CONNOISSEURS AND BEACH BUMS, ONE WEEKEND AT A TIME. Africa, is an imposing structure in the Arabian Sea. The marine fort has a BY EDWINA D’SOUZA distinction of being India’s only undefeated fort on the western coast, in spite of several attacks from the British, Dutch and Shivaji Maharaj of the Maratha Empire. The fort remained unconquered until it became part of the Indian Territory after independence from the British in 1947. The nearby Padmadurg Fort, a protected monument under the Archaeological Survey of India, is equally stunning and can be admired from afar as tourists require special permissions to visit the structure. A morning walk on the Murud beach is pleasantly nice, while evenings bustle with MAHABALESHWAR between Mahabaleshwar and Panchgani, horse rides and speed boats, bhel puri and Mahabaleshwar is the ultimate retreat if and admire the community initiative of corn cob stalls. On the way to Agardanda you want to get away from the crowds of turning local houses into libraries. Jetty, drop by the Khokri village to marvel Mumbai. -

Upsc Current Affairs February- 2020
UNIQUE IAS ACADEMY No.5/45 NVN Layout, New Siddhapudur, Gandhipuram, Coimbatore - 44 UPSC CURRENT AFFAIRS FEBRUARY- 2020 S.NO INDEX PAGE NO GS PAPER – I 1 THE POETS QUOTED IN THE BUDGET SPEECH 1 2 POOMPUHAR: DIGITALLY RECREATED 1 3 VIRTUAL EXPERIENTIAL MUSEUMS 2 4 THE KUMBHABHISHEKAM AT SRI BRIHADEESWARAR TEMPLE 3 5 CHINDU YAKSHAGANAM 4 6 KONARK SUN TEMPLE 4 7 SAMMAKKA SARALAMMA JATARA 5 8 RADIO AND TRIBAL LANGUAGE 6 9 LUI-NGAI-NI FESTIVAL 6 10 MATRIBHASHA DIWAS 6 11 KALA KUMBH 6 12 E-MASIHA APP 7 13 BHARAT-BANGLA TOURISM FESTIVAL 7 14 NATIONAL POPULATION POLICY 7 15 UNITED NATIONS HIGH COMMISSIONER FOR REFUGEES (UNHCR) 8 16 ASSAM ACCORD: CLAUSE 6 8 17 INDONESIA‟S MOUNT MERAPI VOLCANO 9 GS PAPER – II 1 PRESIDENT ADDRESSES JOINT SITTING OF PARLIAMENT 9 2 NATIONAL MEANS-CUM-MERIT SCHOLARSHIP SCHEME (NMMSS) 10 3 SEDITION LAW 10 4 ARTH-GANGA 10 5 RURBAN MISSION 11 6 „CAPACITY BUILDING PROGRAMME FOR ST PRI REPRESENTATIVES‟ AND „1000 12 SPRINGS INITIATIVES‟ 7 SATHI INITIATIVE 12 8 OVERSEAS CITIZEN OF INDIA (OCI) 13 9 MORE TRIBES IN ST CATEGORY IN KARNATAKA 13 10 MEDICAL DEVICES UNDER DRUGS AND COSMETICS ACT 14 11 22ND LAW COMMISSION 15 12 EMPOWERED TECHNOLOGY GROUP 15 13 MAHADAYI TRIBUNAL‟S AWARD 15 14 NATIONAL LEVEL AWARENESS PROGRAMME (NLAP) 2020 17 15 KHELO INDIA UNIVERSITY GAMES 17 16 TELANGANA MUNICIPAL POLLS: FACIAL RECOGNITION APP 17 17 CANDIDATES WITH CRIMINAL HISTORY 18 18 LIVE ATTENUATED CLASSICAL SWINE FEVER (CSF) CELL CULTURE VACCINE 19 19 INDIANS WITH CANCER: WHO REPORT 19 20 WHO NAMES THE NEW CORONA VIRUS: “COVID-19” 20 21 DOMESTIC -

Seat 1 Seat 2 10001 DR. B.R. AMBEDKAR INSTITUTE OF
List of Vacant Seats (Statewise) in Engineering/Technology Stream as on 08.08.2015 College Women's Details of Vacant Seat College Name State Address Unique Id Institute Seat 1 Seat 2 Andaman And Polytechnic Roadpahar 10001 DR. B.R. AMBEDKAR INSTITUTE OF TECHNOLOGY No Vacant Vacant Nicobar Islands Gaonpo Junglighat A.K.R.G. COLLEGE OF ENGINEERING & Nallajerlawest Godavari 10002 Andhra Pradesh No Vacant Vacant TECHNOLOGY Distandhra Pradesh A.M.REDDY MEMORIAL COLLEGE OF Petlurivaripalemnarasaraop 10003 Andhra Pradesh No Vacant Vacant ENGINEERING& TECHNOLOGY etguntur(D.T)A.P Burrripalam 10004 A.S.N.WOMEN S ENGINEERING COLLEGE Andhra Pradesh Road,Nelapadu,Tenali.5222 Yes Vacant Vacant 01,Guntur (Dt), A.P. Nh- 10005 A.V.R & S.V.R ENGINEERING COLLEGE Andhra Pradesh 18,Nannur(V)Orvakal(M),Ku No Vacant Vacant rnool(Dt)518002. A1 GLOBAL INSTITUTE OF ENGINEERING & Markapur, Prakasam 10006 Andhra Pradesh No Vacant Vacant TECHNOLOGY District, Andhra Pradesh. China Irlapadu, Kandukur ABR COLLEGE OF ENGINEERING AND 10007 Andhra Pradesh Road,Kanigiri,Prakasam Dt, No Vacant Vacant TECHNOLOGY Pin 523230. D-Agraharam Villagerekalakunta, Bramhamgari Matam 10008 ACHARYA COLLEGE OF ENGINEERING Andhra Pradesh No Vacant Vacant Mandal,Near Badvel, On Badvel-Mydukur Highwaykadapa 516501 Nh-214Chebrolugollaprolu 10009 ADARSH COLLEGE OF ENGINEERING Andhra Pradesh Mandaleast Godavari No Vacant Vacant Districtandhra Pradesh Valasapalli 10010 ADITYA COLLEGE OF ENGINEERING Andhra Pradesh Post,Madanapalle,Chittoor No Filled Vacant Dist,Andhra Pradesh Aditya Engineering -

JOIN Us : 8830115524 1
JOIN Us : https://targetupsc.in/ 8830115524 1 JOIN Us : https://targetupsc.in/ 8830115524 2 Contents Indian Polity Data Governance Quality Index ( DGQI ): NITI Aayog 18 New rules for postal ballot 19 Air India One 19 Lokpal and Lokayukta 20 Single SBI branch for all FCRA accounts 22 Inter-State River Water Disputes 22 Gupkar Declaration 24 Autonomous District Councils 24 Supreme Court on Right to Protest 25 New H-1B curbs 25 Google to face antitrust case in India 26 Gorkhaland Territorial Administration 26 Television Rating Points(TRP) 26 Who is a declared foreigner? 27 National Commission for Protection of Child Rights 27 Types of Political Parties 28 Right to Information (RTI) Act 29 Right to Information Act, 2005 30 Commitment to Reducing Inequality (CRI) Index, 2020 32 How Election Commission decides on party symbols 33 Diminishing role of Parliament: 33 Gurkha Rights in Assam 35 Saral Jeevan Bima 35 Pradhan Mantri Jivan Jyoti Bima Yojana 36 State Water Grid to overcome flood crisis 36 National Company Law Appellate Tribunal 39 Rationalisation of Autonomous Institutions 39 Sansad Adarsh Gram Yojana (SAGY) 41 Chapter Proceedings 41 JOIN Us : https://targetupsc.in/ 8830115524 3 Chief Information Commissioner 41 District Development Councils (DDC) 42 Election Commission sets up panel to revise Expenditure Limit for candidates 42 Integrity Pact in govt. Organisations 43 Central Vigilance Commission 44 Draft Personal Data Protection Bill, 2019 44 Model Code of Conduct: EC 45 land laws for the Jammu and Kashmir 45 Unlawful Activities (Prevention) -

Morphometric Analysis and Prioritization of Sub-Watersheds in Watershed of Nashik, Maharashtra of Godavari River Basin Using Remote Sensing and GIS As a Tool
International Journal of Innovative Technology and Exploring Engineering (IJITEE) ISSN: 2278-3075, Volume-9 Issue-4, February 2020 Morphometric Analysis and Prioritization of Sub-Watersheds in Watershed of Nashik, Maharashtra of Godavari River Basin using Remote Sensing and GIS as a Tool. Gaurav Kumar, Pratibha Warwade, Gulab Mukund, Nitish Dhaked, Shailesh Suman Abstract: Hydrological analysis and for the integrated I. INTRODUCTION development of the watershed, it is essential to do geomorphometric analysis. A comprehensive study of drainage A naturally occurring hydrological unit which is generally pattern, topography, and most essential erosion status can be defined by its natural boundaries and designated by similar analyzed through morphometric analysis. Prioritization of climatic, physical and topographic conditions is known as a sub-watersheds according to the capacity of the water table of watershed. It is an area from which runoff from precipitation Nashik district watershed was evaluated by linear, aerial and relief flows through a single outlet and ends in large rivers lakes and aspects. The morphometric analysis has been used for the prioritization of seven sub-watersheds of the Godavari river basin the ocean. It is generally classified based on its geographical in the Nashik district, Maharashtra. Using the Digital Elevation area. The Watershed Atlas, prepared by AIS LUS (1990), Model, the sub-watersheds were delineated in ArcMap 10.4 and which describes the area of the watershed as being less than also few extraction works were done in Erdas Imagine. Drains 500 km2 (±50%).The (National Remote Sensing Agency and their corresponding characteristics including stream length, (1995) has further categorized the watershed as stream order, stream frequency, circulatory ratio, texture ratio, compactness coefficient, elongation ratio, form factor, drainage sub-watershed (30–50 km2), mini-watershed (10–30 km2) density, bifurcation ratio is analyzed and their value has evaluated and micro-watershed (5–10 km2) [1]. -
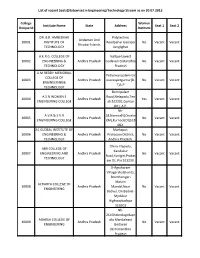
List of Vacant Seats(Statewise) in Engineering/Technology Stream As on 30.07.2015
List of vacant Seats(Statewise) in Engineering/Technology Stream as on 30.07.2015 College Women Institute Name State Address Seat 1 Seat 2 Unique Id Institute DR. B.R. AMBEDKAR Polytechnic Andaman And 10001 INSTITUTE OF Roadpahar Gaonpo No Vacant Vacant Nicobar Islands TECHNOLOGY Junglighat A.K.R.G. COLLEGE OF Nallajerlawest 10002 ENGINEERING & Andhra Pradesh Godavari Distandhra No Vacant Vacant TECHNOLOGY Pradesh A.M.REDDY MEMORIAL Petlurivaripalemnar COLLEGE OF 10003 Andhra Pradesh asaraopetguntur(D. No Vacant Vacant ENGINEERING& T)A.P TECHNOLOGY Burrripalam A.S.N.WOMEN S Road,Nelapadu,Ten 10004 Andhra Pradesh Yes Vacant Vacant ENGINEERING COLLEGE ali.522201,Guntur (Dt), A.P. Nh- A.V.R & S.V.R 18,Nannur(V)Orvaka 10005 Andhra Pradesh No Vacant Vacant ENGINEERING COLLEGE l(M),Kurnool(Dt)518 002. A1 GLOBAL INSTITUTE OF Markapur, 10006 ENGINEERING & Andhra Pradesh Prakasam District, No Vacant Vacant TECHNOLOGY Andhra Pradesh. China Irlapadu, ABR COLLEGE OF Kandukur 10007 ENGINEERING AND Andhra Pradesh No Vacant Vacant Road,Kanigiri,Prakas TECHNOLOGY am Dt, Pin 523230. D-Agraharam Villagerekalakunta, Bramhamgari Matam ACHARYA COLLEGE OF 10008 Andhra Pradesh Mandal,Near No Vacant Vacant ENGINEERING Badvel, On Badvel- Mydukur Highwaykadapa 516501 Nh- 214Chebrolugollapr ADARSH COLLEGE OF olu Mandaleast 10009 Andhra Pradesh No Vacant Vacant ENGINEERING Godavari Districtandhra Pradesh List of vacant Seats(Statewise) in Engineering/Technology Stream as on 30.07.2015 Valasapalli ADITYA COLLEGE OF Post,Madanapalle,C 10010 Andhra Pradesh No Vacant Vacant ENGINEERING hittoor Dist,Andhra Pradesh Aditya Engineering Collegeaditya Nagar, Adb Road, ADITYA ENGINEERING Surampalem,Gande 10011 Andhra Pradesh No Vacant Vacant COLLEGE palli Mandal, East Godavari District, Pin - 533 437, Andhra Pradesh. -

Download Clinically Relevant Somatic and Germline Variation Data from Indian Oral Cancer Patients
INDEX 1. ECONOMY 1. BharatNet Project 08 2. Cabinet Extends Agri Infra Fund loans to APMCs 09 3. Periodic Labour Force Survey (2019 –2020) 10 4. UK Court declares Vijay Mallya Bankrupt 11 2. 1. ECONOMY SNIPPETS 1. NATRAX 13 2. Four Years of GST Regime 14 3. Telecom Duopoly 15 4. Heat Pumps 17 5. Anti-methanogenic feed supplement ‘Harit Dhara’ 18 6. Authorised Economic Operators 19 7. Bhalia Variety of Wheat 20 8. Project BOLD 21 9. New Ministry of Cooperation 22 10. India Industrial Land Bank (IILB) 24 11. India’s First LNG Facility Plant 25 12. SEBI needs to adopt dual Approval System for Independent Directors 25 13. New Solar Project 27 14. Livestock sector 28 15. World Youth Skill Day 29 16. Curbs on Mastercard 30 17. Bad Bank launched for stressed assets 30 18. One District One Focus Product Scheme 32 19. Nord Stream 2 Pipeline (NS2P) project 32 20. Essential Defence Services Bill, 2021 33 21. Economic Liberalisation 34 22. National Farmers Database 35 www.iasgatewayy.comwww. Vetriiias.com / www.iasgatewayy.com Daily Current044-26265326/9884421666/9884472636 Affairs | Monthly Magazines | Online Tests 2 | P a g e 2. ENVIRONMENT 1. What is Gross Environment Product? 37 2. Srivilliputhur Sanctuary has the Highest leopard Density in Western Ghats 38 3. Water Pollution 40 4. Amazon Forest 42 5. Flash Flood and Landslide 43 6. Gecko Species 44 7. Alps Mountain 45 8. Centre launches Secured Logistics Document Exchange (SLDE) 45 9. Kendu Leaf 47 2.1. ENVIRONMENT SNIPPETS 1. Pyrostria Laljii 48 2. Green Hydrogen 48 3. -

An Updated Checklist of the Orchids of Maharashtra, India
LANKESTERIANA 18(1): 23–62. 2018. doi: http://dx.doi.org/10.15517/lank.v18i1.32699 AN UPDATED CHECKLIST OF THE ORCHIDS OF MAHARASHTRA, INDIA JEEWAN SINGH JALAL* & J. JAYANTHI Botanical Survey of India, Western Regional Centre, Pune – 411 001, Maharashtra, India *Corresponding author’s email: [email protected] ABSTRACT. A checklist of the Orchidaceae of Maharashtra is presented based on herbarium collections and recent botanical explorations. This list comprises 32 genera with 106 taxa. The best represented genus is Habenaria with 23 species, followed by Dendrobium with eleven, Eulophia and Oberonia with seven and Peristylus with six. In Maharashtra, the total endemic orchid species are 41 spread over in 13 genera. Of these 30 species, six species are endemic to Peninsular India and five species are endemic to India. Generally orchids are found in the nine major habitats in Maharashtra of which the best representation was observed in the semi-evergreen forests (55 species) followed by moist deciduous forests (48 species), high altitude rocky plateaus (26 species) and dry deciduous forests (25 species). Flowering, habit, habitat, endemic status and distribution in Maharashtra are presented in the checklist. KEY WORDS: India, Maharashtra, orchids, Western Ghats Introduction. The Maharashtra state of the Western (1907) described 31 genera and 75 species of orchids India is known for its flowering plant wealth was from Bombay Presidency. Subsequently, Blatter and explored since 18th century by Law, Graham, Nimmo, McCann (1931a, b, c, 1932) revised the orchid flora Sykes, Jacquemont, Gibson and Dalzell, followed of Bombay Presidency and documented 132 species by Birdwood, Nairne, Hallberg, Acland, Cooke, under 37 genera including many endemic orchids. -

Reg. No Name in Full Residential Address Gender Contact No. Email Id REMARK
Reg. No Name in Full Residential Address Gender Contact No. Email id REMARK 55001 BHUREWAR RAJARAM 16/1, HALI (VADGAON Male 9881535351 BABURAO RASTA) HALI, TAL-UDGIR DIST-LATUR 413518 LATUR Maharashtra 55002 MATTA FARHANAAZ 5TH FLOOR, R.NO. 504, R4 Female 9035833990 [email protected] ZUBAIR 75/77 C-BLOCK, BABULA TANK RD NR. J.J. HOSPITAL, MUMBAI 400009 MUMBAI Maharashtra 55003 DEOKATE RUPALI 4725, KUMBHAR VASTI Female 9096314395 DNYANDEO PANHALE VASTI, NIRAWAGAJ, TAL-BARAMATI DIST-PUNE 413102 PUNE Maharashtra 55004 BERAD SEEMA MAHENDRA 137, MATOSHRI NIWAS, NR. Female 9822503675 MIRGANE HOSP. NAGAR MIRGANE HOSP. NAGAR SOLAPUR RD, DAREWADI 414002 AHMEDNAGAR Maharashtra 55005 SARTALE PRANITA PRATIK, CHANDRASEN Female 9595813523 PRAKASH DESHMUKH C/O- SUBHASHCHANDRA RATHOD RH NO-3, RAMTARA HSG SOCY 431001 AURANGABAD Maharashtra 55006 PATIL KAMLESH DHANRAJ FLAT NO.223, SAGAR Female 8983330308 [email protected] DARSHAN E-WING, OPP BANK OF MAHARASHTR PARNAKA, DAHANU(W) 401601 THANE Maharashtra 55007 BADAKH PRATIBHA A/P-KARAJGAON, TAL- Female 02427/232266 9890039159 DNYANDEO NEWASA DIST-AHMEDNAGAR 414105 AHMEDNAGAR Maharashtra 55008 SAPKAL SWATI EKNATH PANCHAL BLDG, H.NO. 31 Female 020/24213871 [email protected] BIBVEWADIGAON, PUNE 37 411037 PUNE Maharashtra 55009 AGRAWAL ROSHANI BAJERIYA SQUARE MASOBA Female 0712/2762577 9579543576 OMPRAKASH LANE, NAGPUR 440018 NAGPUR Maharashtra 55010 YADAV PRIYAMVADA ROOM NO. 12, MANIKA Female 02226878037 9324925023 AMIRCHAND YADAV CHAWL, MULGAON DONGARI, A.K. RD, ANDHERI(E), MUMBAI 400093 MUMBAI -

44355536003.Pdf
Lankesteriana ISSN: 1409-3871 Lankester Botanical Garden, University of Costa Rica Singh Jalal, Jeewan; Jayanthi, J. An updated checklist of the orchids of Maharashtra, India Lankesteriana, vol. 18, no. 1, 2018, January-April, pp. 23-62 Lankester Botanical Garden, University of Costa Rica DOI: 10.15517/lank.v18i1.32699 Available in: http://www.redalyc.org/articulo.oa?id=44355536003 How to cite Complete issue Scientific Information System Redalyc More information about this article Network of Scientific Journals from Latin America and the Caribbean, Spain and Journal's webpage in redalyc.org Portugal Project academic non-profit, developed under the open access initiative LANKESTERIANA 18(1): 23–62. 2018. doi: http://dx.doi.org/10.15517/lank.v18i1.32699 AN UPDATED CHECKLIST OF THE ORCHIDS OF MAHARASHTRA, INDIA JEEWAN SINGH JALAL* & J. JAYANTHI Botanical Survey of India, Western Regional Centre, Pune – 411 001, Maharashtra, India *Corresponding author’s email: [email protected] ABSTRACT. A checklist of the Orchidaceae of Maharashtra is presented based on herbarium collections and recent botanical explorations. This list comprises 32 genera with 106 taxa. The best represented genus is Habenaria with 23 species, followed by Dendrobium with eleven, Eulophia and Oberonia with seven and Peristylus with six. In Maharashtra, the total endemic orchid species are 41 spread over in 13 genera. Of these 30 species, six species are endemic to Peninsular India and five species are endemic to India. Generally orchids are found in the nine major habitats in Maharashtra of which the best representation was observed in the semi-evergreen forests (55 species) followed by moist deciduous forests (48 species), high altitude rocky plateaus (26 species) and dry deciduous forests (25 species).