Recommended Nomenclature for Zeolite Minerals: Repoft Ofthe
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Mineral Processing
Mineral Processing Foundations of theory and practice of minerallurgy 1st English edition JAN DRZYMALA, C. Eng., Ph.D., D.Sc. Member of the Polish Mineral Processing Society Wroclaw University of Technology 2007 Translation: J. Drzymala, A. Swatek Reviewer: A. Luszczkiewicz Published as supplied by the author ©Copyright by Jan Drzymala, Wroclaw 2007 Computer typesetting: Danuta Szyszka Cover design: Danuta Szyszka Cover photo: Sebastian Bożek Oficyna Wydawnicza Politechniki Wrocławskiej Wybrzeze Wyspianskiego 27 50-370 Wroclaw Any part of this publication can be used in any form by any means provided that the usage is acknowledged by the citation: Drzymala, J., Mineral Processing, Foundations of theory and practice of minerallurgy, Oficyna Wydawnicza PWr., 2007, www.ig.pwr.wroc.pl/minproc ISBN 978-83-7493-362-9 Contents Introduction ....................................................................................................................9 Part I Introduction to mineral processing .....................................................................13 1. From the Big Bang to mineral processing................................................................14 1.1. The formation of matter ...................................................................................14 1.2. Elementary particles.........................................................................................16 1.3. Molecules .........................................................................................................18 1.4. Solids................................................................................................................19 -

A Review of the Structural Architecture of Tellurium Oxycompounds
Mineralogical Magazine, May 2016, Vol. 80(3), pp. 415–545 REVIEW OPEN ACCESS A review of the structural architecture of tellurium oxycompounds 1 2,* 3 A. G. CHRISTY ,S.J.MILLS AND A. R. KAMPF 1 Research School of Earth Sciences and Department of Applied Mathematics, Research School of Physics and Engineering, Australian National University, Canberra, ACT 2601, Australia 2 Geosciences, Museum Victoria, GPO Box 666, Melbourne, Victoria 3001, Australia 3 Mineral Sciences Department, Natural History Museum of Los Angeles County, 900 Exposition Boulevard, Los Angeles, CA 90007, USA [Received 24 November 2015; Accepted 23 February 2016; Associate Editor: Mark Welch] ABSTRACT Relative to its extremely low abundance in the Earth’s crust, tellurium is the most mineralogically diverse chemical element, with over 160 mineral species known that contain essential Te, many of them with unique crystal structures. We review the crystal structures of 703 tellurium oxysalts for which good refinements exist, including 55 that are known to occur as minerals. The dataset is restricted to compounds where oxygen is the only ligand that is strongly bound to Te, but most of the Periodic Table is represented in the compounds that are reviewed. The dataset contains 375 structures that contain only Te4+ cations and 302 with only Te6+, with 26 of the compounds containing Te in both valence states. Te6+ was almost exclusively in rather regular octahedral coordination by oxygen ligands, with only two instances each of 4- and 5-coordination. Conversely, the lone-pair cation Te4+ displayed irregular coordination, with a broad range of coordination numbers and bond distances. -

Mineral of the Month Club November 2015
Mineral of the Month Club November 2015 FLUORITE with CALCITE This month we are featuring composite specimens of fluorite with calcite from China’s Xianghualing Mine. Our write-up discusses the mine’s history, the industrial importance of fluorite, and an internationally recognized collector’s guidelines for assessing the visual beauty of mineral specimens. OVERVIEW PHYSICAL PROPERTIES Chemistry: Calcium Fluoride CaF2 Class: Halides Group: Fluorite Crystal System: Isometric (Cubic) Crystal Habits: Usually cubic, often as penetration twins; less frequently octahedral; rarely dodecahedral; also occurs in botryoidal, granular, massive, earthy, and columnar forms. Color: White, colorless, violet, purple, lilac, blue, green, yellow, brown, amber, bluish- black, pink, and rose-red; the “rainbow” variety is multicolored. Luster: Vitreous Transparency: Transparent to translucent Streak: White Cleavage: Perfect in four directions, forming octahedrons. Fracture/Tenacity: Uneven; brittle. Hardness: 4.0 Specific Gravity: 3.0-3.2 Luminescence: Often fluorescent and phosphorescent, sometimes thermoluminescent and triboluminescent. Refractive Index: 1.433 Distinctive Features and Tests: Best field indicators are well-developed cubic or octahedral crystals; perfect, four-directional cleavage and a tendency to cleave into octahedrons; relative softness; and occurrence in fluorine-rich, mineralogical environments. Dana Classification Number: 9.2.1.1 NAME: The word “fluorite,” pronounced FLOR-ite, stems from the Latin fluor, meaning “flow” and alluding -

Shin-Skinner January 2018 Edition
Page 1 The Shin-Skinner News Vol 57, No 1; January 2018 Che-Hanna Rock & Mineral Club, Inc. P.O. Box 142, Sayre PA 18840-0142 PURPOSE: The club was organized in 1962 in Sayre, PA OFFICERS to assemble for the purpose of studying and collecting rock, President: Bob McGuire [email protected] mineral, fossil, and shell specimens, and to develop skills in Vice-Pres: Ted Rieth [email protected] the lapidary arts. We are members of the Eastern Acting Secretary: JoAnn McGuire [email protected] Federation of Mineralogical & Lapidary Societies (EFMLS) Treasurer & member chair: Trish Benish and the American Federation of Mineralogical Societies [email protected] (AFMS). Immed. Past Pres. Inga Wells [email protected] DUES are payable to the treasurer BY January 1st of each year. After that date membership will be terminated. Make BOARD meetings are held at 6PM on odd-numbered checks payable to Che-Hanna Rock & Mineral Club, Inc. as months unless special meetings are called by the follows: $12.00 for Family; $8.00 for Subscribing Patron; president. $8.00 for Individual and Junior members (under age 17) not BOARD MEMBERS: covered by a family membership. Bruce Benish, Jeff Benish, Mary Walter MEETINGS are held at the Sayre High School (on Lockhart APPOINTED Street) at 7:00 PM in the cafeteria, the 2nd Wednesday Programs: Ted Rieth [email protected] each month, except JUNE, JULY, AUGUST, and Publicity: Hazel Remaley 570-888-7544 DECEMBER. Those meetings and events (and any [email protected] changes) will be announced in this newsletter, with location Editor: David Dick and schedule, as well as on our website [email protected] chehannarocks.com. -

NEW MINERAL NAMES Mrcn,Q.Br, Frprscubn Liberite
THE AMERICAN MINERALOGIST, VOL 50, MARCH APRIL, 1965 NEW MINERAL NAMES Mrcn,q.Br,FrprscuBn Liberite Cn'ur+-LrN Cnao, Liberite (Li, Be SiOa),a new lithium-beryllium silicate mineral from the Nanling Ranges, South China: Ti Chih llsueh Pao 4 (3),33+,342 (1964); Chem. 4bs.61,15841(1964). Analysis gave SiO248.39, AlrO30 26, Fe2O30.11, MgO 0.49, BeO 25.47,LirO 23.43, CaO 029, NazO 025, KzO 0.10, HrO 0.65, sum 99.44o/a,corresponding to Li2BeSiOa.X-ray powder data gave the strongest lines as 3.799 (lO),3.709 (10),2.581(10), 1.M7 (10), 1.233 (10). The unit cell has a ?, b 4 9464, c 8.53954. The mineral occurs as minute mono- clinic aggregates occasionally showing pinacoidal faces, color pale-yellow to brown, cleavage (010) perfect, (100) and (001) distinct, luster vitreous on cleavages,slightly oily on fractures, brittle, H 7.0875 (??, MF), G 2.688+0.001. Biaxial (-), with zs (Na) q 7.6220,9 1.6332,y1.6380,2V66o18',ZAc:4lo,elongation positive.The mineral occurs in association with lepidolite, natrolite, eucryptite, hsianghualite (Am. Minerat.44, 1327; 46,244), cassiterite, and scheelitein veins cutting ribbon rock (lepidolite-fluorite-magnet- ite) in tactites. The name is presumabiy for the composition. Sederholmite, W'ilkmanite, Kullerudite, M?ikinenite and Triistedtite Y. Vuonrr.ernrn, A. Hunlre ewo A. HAxrr, Sederholmite, wilkmanite, kullerudite, miikinenite, and triistedtite, five new nickel selenide minerals. Compt. rmdus Soc.geol,. Finlanile 36, 113-125 (1964). The new selenium minerals occur in sills of albite diabase in a schist formation, asso- ciated with low-grade uranium mineralization at Kuusamo, northeast Finland. -

Li0.5Al0.5)Al2o4, a New Lithium Oxyspinel Mineral from The
This is the peer-reviewed, final accepted version for American Mineralogist, published by the Mineralogical Society of America. The published version is subject to change. Cite as Authors (Year) Title. American Mineralogist, in press. DOI: https://doi.org/10.2138/am-2021-7932. http://www.minsocam.org/ 49 50 Chukochenite (Li0.5Al0.5)Al2O4, a new lithium oxyspinel mineral from the 51 Xianghualing skarn, Hunan Province, China 52 53 CAN RAO1*, XIANGPING GU2, RUCHENG WANG3, QUNKE XIA1, YUANFENG CAI3, CHUANWAN 54 DONG1, FRÉDÉRIC HATERT4, AND YANTAO HAO1 55 56 1 Key Laboratory of Geoscience Big Data and Deep Resource of Zhejiang Province, School of Earth 57 Sciences, Zhejiang University, Hangzhou 310027, China 58 2 School of Earth Sciences and Info-physics, Central South University, Changsha, 410083, China 59 3 State Key Laboratory for Mineral Deposits Research, School of Earth Sciences and Engineering, Nanjing 60 University, Nanjing, 210046, China 61 4 Laboratoire de Minéralogie, B18, Université de Liège, B-4000 Liège, Belgium 62 *E-mail: [email protected] 63 64 ABSTRACT 65 Chukochenite, (Li0.5Al0.5)Al2O4, is a new mineral species from the Xianghualing skarn, 66 Hunan Province, southern China. It occurs as subhedral to euhedral crystals up to 200 μm 67 across in the green rock of Xianghualing skarn, closely associated with fluorite, phlogopite, 68 chrysoberyl, margarite, chlorite, ferronigerite-2N1S, and zinconigerite-2N1S. The crystals are 69 colorless and transparent with vitreous luster. Chukochenite is brittle with irregular fracture, 70 has a Mohs hardness of 8, and shows light red fluorescence under 253.7 nm UV radiation and 71 light green fluorescence under 365 nm UV radiation. -

Gold, (Au) Occurrence: Gold Is a Rare Mineral, but Is Widely Distributed in Low by Julian C
Tips and Trips Page 7 The Georgia Mineral Society February 2005 Gold, (Au) Occurrence: Gold is a rare mineral, but is widely distributed in low By Julian C. Gray, concentrations. It occurs most frequently as a native Mineral Section Chair metal, but may combine with selenium or telluride. It is most commonly associated with high silica igneous rocks such as granite and granodiorite. Boyle (1987) uses the following classification of gold occurrences: (1) Auriferous porphyry dikes, sills, and stocks; auriferous pegmatites, coarse-grained granitic bodies, aplites, and Gold albitites Plumbago Mine Sierra County, Generally Precambrian occurrences, these rocks California have low (0.003 parts per million - ppm) gold 12 mm x 7 mm concentrations. Auriferous sulfides, pyrite and pyrrhotite, may contain 0.10 ppm gold and 1 ppm (Photo By Julian silver. These deposits are rarely economical. C. Gray) (2) Carbonatites and carbonatite-related bodies Carbonatites are multiphase ultramafic (high iron and magnesium) and carbonate intrusions, usually in a ring-shaped geometry. Although the carbonatites have low concentrations of gold, the late hydrothermal stage may be slightly enriched in gold and silver. The silver occurs in argentiferous galena Name: and tetrahedrite, and the gold is found as native gold Known from ancient times. German: Geld and in association with pyrite, pyrrhotite, molybdenite, Sanskrit: jyal. Aurum is the Greek word for gold from chalcopyrite, and other copper sulfides. which we obtain the chemical symbol, Au. This is also the root of the modifier, auriferous, which means These deposits are also rarely economical. Two that a mineral contains some gold: auriferous pyrite known deposits are the Olympic Dam (Roxby Downs) or auriferous galena, for instance. -

IMA–CNMNC Approved Mineral Symbols
Mineralogical Magazine (2021), 85, 291–320 doi:10.1180/mgm.2021.43 Article IMA–CNMNC approved mineral symbols Laurence N. Warr* Institute of Geography and Geology, University of Greifswald, 17487 Greifswald, Germany Abstract Several text symbol lists for common rock-forming minerals have been published over the last 40 years, but no internationally agreed standard has yet been established. This contribution presents the first International Mineralogical Association (IMA) Commission on New Minerals, Nomenclature and Classification (CNMNC) approved collection of 5744 mineral name abbreviations by combining four methods of nomenclature based on the Kretz symbol approach. The collection incorporates 991 previously defined abbreviations for mineral groups and species and presents a further 4753 new symbols that cover all currently listed IMA minerals. Adopting IMA– CNMNC approved symbols is considered a necessary step in standardising abbreviations by employing a system compatible with that used for symbolising the chemical elements. Keywords: nomenclature, mineral names, symbols, abbreviations, groups, species, elements, IMA, CNMNC (Received 28 November 2020; accepted 14 May 2021; Accepted Manuscript published online: 18 May 2021; Associate Editor: Anthony R Kampf) Introduction used collection proposed by Whitney and Evans (2010). Despite the availability of recommended abbreviations for the commonly Using text symbols for abbreviating the scientific names of the studied mineral species, to date < 18% of mineral names recog- chemical elements -

14 Non-Pegmatitic Deposits of Beryllium: Mineralogy, Geology, Phase Equilibria and Origin Mark D
14 Non-pegmatitic Deposits of Beryllium: Mineralogy, Geology, Phase Equilibria and Origin Mark D. Barton and Steven Young Center for Mineral Resources Department of Geosciences University of Arizona Tucson, Arizona 85721 [email protected] INTRODUCTION Non-pegmatitic occurrences of Be minerals constitute a diverse set of geologic environments of considerable mineralogical and petrological interest; they currently provide the majority of the world’s Be ore and emeralds and they contain the greatest resource of these commodities. Of the approximately 100 Be minerals known (see Chapter 1 by Grew; Appendix A), most occur in hydrothermal deposits or non-pegmatitic igneous rocks, where their distribution varies systematically with the setting and origin (Table 1, Fig. 1). Figure 1. Chemography of the principal solid phases in the BeO-Al2O3-SiO2-H2O(-F2O-1) “BASH” system with the projected positions of helvite group and alkali Be silicates. Also shown are generalized fields for some of the major types natural of occurrences (cf. Table 1, Fig. 4; see text for discussion). Beryllium minerals are best known from geologic systems associated with felsic magmatism. They also occur in a variety of settings that lack evident igneous associations. Environments range from the surface to the deep crust and host rocks range from feldspathic to carbonate to ultramafic in composition. Genetically related igneous rocks are felsic and share low calcium and high F contents, but are diverse in composition, setting and origin. Compositions range from strongly peraluminous to 1529-6466/02/0050-0014$10.00 DOI:10.2138/rmg.2002.50.14 592 Chapter 14: Barton & Young Table 1. -
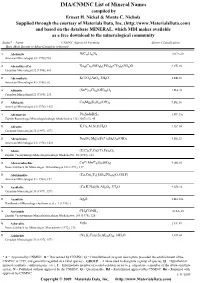
IMA/CNMNC List of Mineral Names
IMA/CNMNC List of Mineral Name s compiled by Ernest H. Nickel & Monte C. Nichols Supplied through the courtesy of Materials Data, Inc. (http://www.MaterialsData.com) and based on the database MINERAL, which MDI makes available as a free download to the mineralogical community Status* Name CNMNC Approved Formula Strunz Classification Best, Most Recent or Most Complete reference. A Abelsonite NiC£¡H£¢N¤ 10.CA.20 American Mineralogist 63 (1978) 930 A Abenakiite-(Ce) Na¢¦Ce¦(SiO£)¦(PO¤)¦(CO£)¦(SO¢)O 9.CK.10 Canadian Mineralogist 32 (1994), 843 G Abernathyite K(UO¢)AsO¤•3H¢O 8.EB.15 American Mineralogist 41 (1956), 82 A Abhurite (SnÀÈ)¢¡Cl¡¦(OH)¡¤O¦ 3.DA.30 Canadian Mineralogist 23 (1985), 233 D Abkhazite Ca¢Mg¥Si¨O¢¢(OH)¢ 9.DE.10 American Mineralogist 63 (1978), 1023 A Abramovite Pb¢SnInBiS§ 2.HF.25a Zapiski Rossiiskogo Mineralogicheskogo Obshchetstva 136 (2007) (5), 45 D Abrazite K,Ca,Al,Si,O,H¢O 9.GC.05 Canadian Mineralogist 35 (1997), 1571 D Abriachanite Na¢(Fe,Mg)£(FeÁÈ)¢Si¨O¢¢(OH)¢ 9.DE.25 American Mineralogist 63 (1978), 1023 D Absite (U,Ca,Y,Ce)(Ti,Fe)¢O¦ Zapiski Vsesoyuznogo Mineralogicheskogo Obshchestva 92 (1963), 113 A Abswurmbachite CuÀÈ(MnÁÈ)¦O¨(SiO¤) 9.AG.05 Neues Jahrbuch für Mineralogie, Abhandlungen 163 (1991), 117 D Abukumalite (Ca,Ce)¢Y£(SiO¤,PO¤)£(O,OH,F) American Mineralogist 51 (1966), 152 D Acadialite (Ca,K,Na)(Si,Al)£O¦•3H¢O 9.GD.10 Canadian Mineralogist 35 (1997), 1571 G Acanthite Ag¢S 2.BA.30a Handbook of Mineralogy (Anthony et al.), 1 (1990), 1 A Acetamide CH£CONH¢ 10.AA.20 Zapiski Vsesoyuznogo Mineralogicheskogo -

Urvey Note Volume 23, Number 3 Fall 1989 Utah Geological & Mineral S Ur V E: Y
URVEY NOTE VOLUME 23, NUMBER 3 FALL 1989 UTAH GEOLOGICAL & MINERAL S UR V E: Y photo credit: Ron Surdam, Dept. of Geology, University of Wyoming TABLE OF CONTENTS Zeolite Occurrences of Utah • • • . • • 2 THE DIRECTOR'S Utah Earthquake Activity •••••••••• 13 Teacher's Corner •. ...••• •• . • •••• 14 byM. Lee Allison Allison Named UGMS Direc tor . • .. • . 15 PERSPECTIVE Staff Changes •• •• .... • •• ••• . •• •. 15 Books & Papers ..• •• . • .... • •• • •• 16 Tooele 1° x 2° Mineral Occ urrence Map •..•.. ••••••• . 17 UGMS Industrial Mineral Q n October of this year Genevieve Atwood step- Publications ••. .• • .• •• • • •.... • 18 ped down after eight years as Director of UGMS Saline Resources of Utah ... •• • .. .. 21 to pursue educational and other career goals. In Last Map Ceremonies • ......•• • • • . 31 doing so, UGMS lost an incredibly dynamic, out Recent UGMS Publicatio ns .. .... ••. 32 spoken, innovative leader who broadened the scope of the Survey's role in understanding geologic hazards and mapping the geology of the state. It will be STATE OF UTAH NORMAN H . BANGERTER, GOVERNOR difficult to fill her shoes. DEPARTMENT OF NATURAL RESOURCES The transition period from announcement of my appointment as her replace DEE C. HANSEN, EXECUTIVE DIRECTOR ment to the day I actually took over was remarkably smooth as Genevieve so ught and accepted my recommendations on budget requests for the coming fiscal SURVEY NOTES STAFF year, and consulted me on all major managerial decisions. It was clear that her EDITOR J. STRIN GFELLOW concerns are with the hea lth and vitality of the UGMS and not with the fact that I EDITORIAL STAFF would be changing some aspects of her programs. I thank her and jo in with the Julia M. -

Crystal Chemistry of Beryllium
Crystal Chemistry of Beryllium 5 GEOLOGICAL SURVEY PROFESSIONAL PAPER 468 Crystal Chemistry of Beryllium By MALCOLM ROSS GEOLOGICAL SURVEY PROFESSIONAL PAPER 468 A detailed discussion of the crystal chemistry of all the known beryllium minerals UNITED STATES GOVERNMENT PRINTING OFFICE, WASHINGTON : 1964 UNITED STATES DEPARTMENT OF THE INTERIOR STEW ART L. UDALL, Secretary GEOLOGICAL SURVEY Thomas B. Nolan, Director The U.S. Geological Survey Library has cataloged this publication as follows: Ross, Malcolm, 1929- Crystal chemistry of beryllium. Washington, U.S. Govt. Print. Off., 1964. iv, 30 p. illus., diagrs., tables. 29 em. (U.S. Geological Survey. Professional paper 468) Bibliography : p. 28-30. 1. Beryllium. 2. Crystallization. I. Title. (Series) For safe by the Superintendent of Documents, U.S. Government Printing Office Washington, D.C. 20402 CONTENTS Page Page Abstract __________________________________________ _ 1 The beryllium minerals-continued 16 Introduction ______________________________________ _ 1 Gu~aite ________ ------------------------------- The beryllium minerals _____________________________ _ leucophanite _ -- __ ------------------------- 16 1 16 Amino.ffite ____________________________________ _ meliphanite __ - __ --------------------------- 2 18 Barylite ______________________________________ _ Milarite ______ --_------------------------------ 2 Moraesite _____________________________________ _ 19 Bavenite ______________________________________ _ 3 Bearsite _____ ---------------------------------- 19 Bertrandite