3. List of Nominal Species of Trichiuroidea
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Checklist of Marine Demersal Fishes Captured by the Pair Trawl Fisheries in Southern (RJ-SC) Brazil
Biota Neotropica 19(1): e20170432, 2019 www.scielo.br/bn ISSN 1676-0611 (online edition) Inventory Checklist of marine demersal fishes captured by the pair trawl fisheries in Southern (RJ-SC) Brazil Matheus Marcos Rotundo1,2,3,4 , Evandro Severino-Rodrigues2, Walter Barrella4,5, Miguel Petrere Jun- ior3 & Milena Ramires4,5 1Universidade Santa Cecilia, Acervo Zoológico, R. Oswaldo Cruz, 266, CEP11045-907, Santos, SP, Brasil 2Instituto de Pesca, Programa de Pós-graduação em Aquicultura e Pesca, Santos, SP, Brasil 3Universidade Federal de São Carlos, Programa de Pós-Graduação em Planejamento e Uso de Recursos Renováveis, Rodovia João Leme dos Santos, Km 110, CEP 18052-780, Sorocaba, SP, Brasil 4Universidade Santa Cecília, Programa de Pós-Graduação de Auditoria Ambiental, R. Oswaldo Cruz, 266, CEP11045-907, Santos, SP, Brasil 5Universidade Santa Cecília, Programa de Pós-Graduação em Sustentabilidade de Ecossistemas Costeiros e Marinhos, R. Oswaldo Cruz, 266, CEP11045-907, Santos, SP, Brasil *Corresponding author: Matheus Marcos Rotundo: [email protected] ROTUNDO, M.M., SEVERINO-RODRIGUES, E., BARRELLA, W., PETRERE JUNIOR, M., RAMIRES, M. Checklist of marine demersal fishes captured by the pair trawl fisheries in Southern (RJ-SC) Brazil. Biota Neotropica. 19(1): e20170432. http://dx.doi.org/10.1590/1676-0611-BN-2017-0432 Abstract: Demersal fishery resources are abundant on continental shelves, on the tropical and subtropical coasts, making up a significant part of the marine environment. Marine demersal fishery resources are captured by various fishing methods, often unsustainably, which has led to the depletion of their stocks. In order to inventory the marine demersal ichthyofauna on the Southern Brazilian coast, as well as their conservation status and distribution, this study analyzed the composition and frequency of occurrence of fish captured by pair trawling in 117 fishery fleet landings based in the State of São Paulo between 2005 and 2012. -

Fishery Bulletin/U S Dept of Commerce National Oceanic
FOOD HABITS OF BAIT-CAUGHT SKIPJACK TUNA, KATSUWONUS PELAMIS, FROM THE SOUTHWESTERN ATLANTIC OCEAN LISA ANKENBRANDTI ABSTRACT Stomach contents of skipjack tuna captured in 1981-82 hy live pole-and-line vessels off the southern coast of Brazil were analyzed for the presence of larval and juvenile skipjack tuna. The percentage frequency of occurrence. percent number. and percent volume were evaluated. Of the 1.041 stomachs that were exam ined for food, 436 were empty. The mean volume of food in all stomachs analyzed was 36.9 mL, of which 18.9 mL was bait and 18.0 mL was prey. The gonostomatid Mau.rolicu8 muelleri and the euphausiid Eupha:usia simil:is were the principal foods. Other important foods were the chub mackerel, Scomber japonicu,; the frigate tuna, A uxi, thazll.rd; gem pylids; trichiurids; and carangids. In the study area, adult skipjack tuna were not found to feed on their young. Kruskall-Wallis nonparametric one-way analysis of variance was used to test for differences in the mean volumetric ratios of food items in relation to skipjack size. The percentage of E. simili, in the diet was found to decrease, while the proportion of M. mu.elleri was found to increase with increasing skipjack size. Seasonal variations in the diet were also examined and discussed. Apparently the anatomy of their gill raker apparatus allows skipjack to ingest a wide variety of prey types above a minimum size. These variations in the food can be attributed to the number and size of the prey species in an area. A Brazilian skipjack pole-and-line fishery has been Duarte-Bello 1961; Klawe 1961; Dragovich 1970; developing in the Rio de Janeiro area since 1979 Dragovich and Potthoff 1972). -

Fao Species Catalogue
FAO Fisheries Synopsis No. 125, Volume 15 ISSN 0014-5602 FIR/S1 25 Vol. 15 FAO SPECIES CATALOGUE VOL. 15. SNAKE MACKERELS AND CUTLASSFISHES OF THE WORLD (FAMILIES GEMPYLIDAE AND TRICHIURIDAE) AN ANNOTATED AND ILLUSTRATED CATALOGUE OF THE SNAKE MACKERELS, SNOEKS, ESCOLARS, GEMFISHES, SACKFISHES, DOMINE, OILFISH, CUTLASSFISHES, SCABBARDFISHES, HAIRTAILS AND FROSTFISHES KNOWN TO DATE 12®lÄSÄötfSE, FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS FAO Fisheries Synopsis No. 125, Volume 15 FIR/S125 Vol. 15 FAO SPECIES CATALOGUE VOL. 15. SNAKE MACKERELS AND CUTLASSFISHES OF THE WORLD (Families Gempylidae and Trichiuridae) An Annotated and Illustrated Catalogue of the Snake Mackerels, Snoeks, Escolars, Gemfishes, Sackfishes, Domine, Oilfish, Cutlassfishes, Scabbardfishes, Hairtails, and Frostfishes Known to Date by I. Nakamura Fisheries Research Station Kyoto University Maizuru, Kyoto, 625, Japan and N. V. Parin P.P. Shirshov Institute of Oceanology Academy of Sciences Krasikova 23 Moscow 117218, Russian Federation FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS Rome, 1993 The designations employed and the presenta tion of material in this publication do not imply the expression of any opinion whatsoever on the part of the Food and Agriculture Organization of the United Nations concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. M -40 ISBN 92-5-103124-X All rights reserved. No part of this publication may be reproduced, stored in a retrieval system, or transmitted in any form or by any means, electronic, mechanical, photocopying or otherwise, without the prior permission of the copyright owner. Applications for such permission, with a statement of the purpose and extent of the reproduction, should be addressed to the Director, Publications Division, Food and Agriculture Organization of the United Nations, Via delle Terme di Caracalla, 00100 Rome, Italy. -

Fao Species Catalogue
FAO Fisheries Synopsis No. 125, Volume 15 ISSN 0014-5602 FIR/S125 Vol. 15 FAO SPECIES CATALOGUE VOL. 15. SNAKE MACKERELS AND CUTLASSFISHES OF THE WORLD (FAMILIES GEMPYLIDAE AND TRICHIURIDAE) AN ANNOTATED AND ILLUSTRATED CATALOGUE OF THE SNAKE MACKERELS, SNOEKS, ESCOLARS, GEMFISHES, SACKFISHES, DOMINE, OILFISH, CUTLASSFISHES, SCABBARDFISHES, HAIRTAILS AND FROSTFISHES KNOWN TO DATE FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS FAO Fisheries Synopsis No. 125, Volume 15 FIR/S125 Vol. 15 FAO SPECIES CATALOGUE VOL. 15. SNAKE MACKERELS AND CUTLASSFISHES OF THE WORLD (Families Gempylidae and Trichiuridae) An Annotated and Illustrated Catalogue of the Snake Mackerels, Snoeks, Escolars, Gemfishes, Sackfishes, Domine, Oilfish, Cutlassfishes, Scabbardfishes, Hairtails, and Frostfishes Known to Date I. Nakamura Fisheries Research Station Kyoto University Maizuru, Kyoto, 625, Japan and N. V. Parin P.P. Shirshov Institute of Oceanology Academy of Sciences Krasikova 23 Moscow 117218, Russian Federation FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS Rome, 1993 The designations employed and the presenta- tion of material in this publication do not imply the expression of any opinion whatsoever on the part of the Food and Agriculture Organization of the United Nations concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. M-40 ISBN 92-5-103124-X All rights reserved. No part of this publication may be reproduced, stored in a retrieval system, or transmitted in any form or by any means, electronic, mechanical, photocopying or otherwise, without the prior permission of the copyright owner. Applications for such permission, with a statement of the purpose and extent of the reproduction, should be addressed to the Director, Publications Division, Food and Agriculture Organization of the United Nations, Via delle Terme di Caracalla, 00100 Rome, Italy. -

Intrinsic Vulnerability in the Global Fish Catch
The following appendix accompanies the article Intrinsic vulnerability in the global fish catch William W. L. Cheung1,*, Reg Watson1, Telmo Morato1,2, Tony J. Pitcher1, Daniel Pauly1 1Fisheries Centre, The University of British Columbia, Aquatic Ecosystems Research Laboratory (AERL), 2202 Main Mall, Vancouver, British Columbia V6T 1Z4, Canada 2Departamento de Oceanografia e Pescas, Universidade dos Açores, 9901-862 Horta, Portugal *Email: [email protected] Marine Ecology Progress Series 333:1–12 (2007) Appendix 1. Intrinsic vulnerability index of fish taxa represented in the global catch, based on the Sea Around Us database (www.seaaroundus.org) Taxonomic Intrinsic level Taxon Common name vulnerability Family Pristidae Sawfishes 88 Squatinidae Angel sharks 80 Anarhichadidae Wolffishes 78 Carcharhinidae Requiem sharks 77 Sphyrnidae Hammerhead, bonnethead, scoophead shark 77 Macrouridae Grenadiers or rattails 75 Rajidae Skates 72 Alepocephalidae Slickheads 71 Lophiidae Goosefishes 70 Torpedinidae Electric rays 68 Belonidae Needlefishes 67 Emmelichthyidae Rovers 66 Nototheniidae Cod icefishes 65 Ophidiidae Cusk-eels 65 Trachichthyidae Slimeheads 64 Channichthyidae Crocodile icefishes 63 Myliobatidae Eagle and manta rays 63 Squalidae Dogfish sharks 62 Congridae Conger and garden eels 60 Serranidae Sea basses: groupers and fairy basslets 60 Exocoetidae Flyingfishes 59 Malacanthidae Tilefishes 58 Scorpaenidae Scorpionfishes or rockfishes 58 Polynemidae Threadfins 56 Triakidae Houndsharks 56 Istiophoridae Billfishes 55 Petromyzontidae -

Title a NEW GENUS and SPECIES of GEMPYLIDAE (PISCES
A NEW GENUS AND SPECIES OF GEMPYLIDAE (PISCES Title : PERCIFORMES) FROM TONGA RIDGE Author(s) Nakamura, Izumi; Fujii, Eiichi PUBLICATIONS OF THE SETO MARINE BIOLOGICAL Citation LABORATORY (1983), 27(4-6): 173-191 Issue Date 1983-01-31 URL http://hdl.handle.net/2433/176057 Right Type Departmental Bulletin Paper Textversion publisher Kyoto University . ~·: ~ ...... A NEW GENUS AND SPECIES OF GEMPYLIDAE (PISCES: PERCIFORMES) FROM TONGA RIDGE'> IzuMI NAKAMURA2> Division of Fishes, Department of Ve~tebrate Zoology, NMNH, Smithsonian Institution and EncHI FUJII Suruga Bay Marine Biological Society, Tokai University With Text:fi.iures 1~13 and Table 1 During the research cruise ofR.V. kaiyo Maru, which; the junior author (EF) joined around the Tonga Ridge area in the South Pacific, in 1977, a number of gempylid fish were caught in one haul of an otter trawl out of five hauls. This gempylid first seemed to be a second species of the ~onotypic genus Thyrsitops, be cause it has a similar appearance and counts to those of Thyrsitops lepidopoides. ·There are. some morphometric differences between the species:. our gempylid has a semi fusiform and robust body, and T. lepidopoides has a much. more compressed and slender body; . Careful study revealed that the gempylid species obtained from .the Tonga Ridge is referable to a new genus and new species and that it has both gempylid and scombrid characteristics as discussed later .. , This species could be a key species in considering interrelationships between Gempylidae and 1Scombridae. Therefore, osteologicaL features are described in detail. Material and Methods Material used in this st~dy ~ere the 38 specimens caught in one trawl haul in the Tonga Ridge area (Fig. -

Length-Weight Relationship of Small Pelagic Fish Species of the Southeast and South Brazilian Exclusive Economic Zone
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Aquatic Commons Length-Weight Relationship of Small Pelagic Fish Species of the Southeast and South Brazilian Exclusive Economic Zone R.A. Bernardes, and C.L.D.B. Rossi-Wongtschowski Abstract The length-weight relationship (LWR) parameters of 23 small pelagic fish species (belonging to 13 families) from the South-Southeast Brazilian Exclusive Economic Zone in 1996 and 1997 are presented. The b values varied between 2.72 and 3.53. The samples for this study were collected during hydroacoustic surveys covering an area of 700 000 km2 Introduction 22 Cabo de Sao Tomé This work is based on data gathered during an assessment of the Santos composition and fishery potential of 24 pelagic resources in the South and Southeast Brazilian Economic 26 Exclusive Zone (EEZ). In spite of good knowledge of the pelagic Itajaí ichthyofauna in the coastal area, 28 there is little information about Cabo de Santa Marta species inhabiting the continental shelf and slope below 100 m depth. Latitude (˚S) 30 Three hydroacoustic surveys were conducted between depths of 100 and 1 500m during 1996 and 1997 32 and covered an area of about 700 2 000 km (Fig. 1). A total of 140 Chuí species of fish belonging to 18 34 genera and 60 families were reported during these surveys 38 (Yamaguti et al. 1999). This 54 52 50 48 46 44 42 40 contribution presents the LWR parameters for 23 of the most Longitude (˚W) abundant species caught. Fig.1. -

ASFIS ISSCAAP Fish List February 2007 Sorted on Scientific Name
ASFIS ISSCAAP Fish List Sorted on Scientific Name February 2007 Scientific name English Name French name Spanish Name Code Abalistes stellaris (Bloch & Schneider 1801) Starry triggerfish AJS Abbottina rivularis (Basilewsky 1855) Chinese false gudgeon ABB Ablabys binotatus (Peters 1855) Redskinfish ABW Ablennes hians (Valenciennes 1846) Flat needlefish Orphie plate Agujón sable BAF Aborichthys elongatus Hora 1921 ABE Abralia andamanika Goodrich 1898 BLK Abralia veranyi (Rüppell 1844) Verany's enope squid Encornet de Verany Enoploluria de Verany BLJ Abraliopsis pfefferi (Verany 1837) Pfeffer's enope squid Encornet de Pfeffer Enoploluria de Pfeffer BJF Abramis brama (Linnaeus 1758) Freshwater bream Brème d'eau douce Brema común FBM Abramis spp Freshwater breams nei Brèmes d'eau douce nca Bremas nep FBR Abramites eques (Steindachner 1878) ABQ Abudefduf luridus (Cuvier 1830) Canary damsel AUU Abudefduf saxatilis (Linnaeus 1758) Sergeant-major ABU Abyssobrotula galatheae Nielsen 1977 OAG Abyssocottus elochini Taliev 1955 AEZ Abythites lepidogenys (Smith & Radcliffe 1913) AHD Acanella spp Branched bamboo coral KQL Acanthacaris caeca (A. Milne Edwards 1881) Atlantic deep-sea lobster Langoustine arganelle Cigala de fondo NTK Acanthacaris tenuimana Bate 1888 Prickly deep-sea lobster Langoustine spinuleuse Cigala raspa NHI Acanthalburnus microlepis (De Filippi 1861) Blackbrow bleak AHL Acanthaphritis barbata (Okamura & Kishida 1963) NHT Acantharchus pomotis (Baird 1855) Mud sunfish AKP Acanthaxius caespitosa (Squires 1979) Deepwater mud lobster Langouste -

Larval Fish Recruitment and Research in the Americas
NOAA Technical Report NMFS 95 January 1991 Larval Fish Recruitment and Research in the Americas Thirteenth Annuol Larval Fish Conference Mbido" Mexico, May 1989 Robert D. Hoyt (editor) u.s. Department of Commerce NOAA Technical Report NMFS _ The major responsibilities of the National Marine Fisheries Service (NMFS) are to monitor and assess the abundance and geographic distribution of fishery resources, to understand and predict fluctuations in the quantity and distribution of these resources, and to establish levels for their optimum use. NMFS is also charged with the development and implementation of policies for managing national fishing grounds, development and enforcement of domestic fisheries regulations, surveillance of foreign fishing off United States coastal waters, and the development and enforcement of international fishery agreements and policies. NMFS also assists the fishing industry through marketing service and economic analysis programs, and mortgage in surance and vessel construction subsidies. It collects, analyzes, and publishes statistics on various phases of the industry. The NOAA Technical Report NMFS series was established in 1983 to replace two subcategories of the Technical Reports series: "Special Scientific Report-Fisheries" and "Circular." The series contains the following types of reports: Scientific investigations that document long-term continuing programs of NMFS; intensive scientific reports on studies of restricted scope; papers on applied fishery problems; technical reports of general interest intended to aid conservation and management; reports that review in considerable detail and at a high technical level certain broad areas of research; and technical papers originating in economics studies and from management investigations. Since this is a formal series, all submitted papers receive peer review and those accepted receive professional editing before publication. -
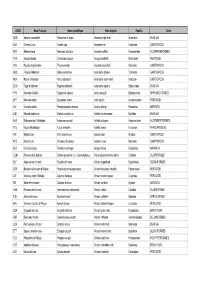
Liste Espèces
CODE Nom Français Nom scientifique Nom Anglais Famille Ordre ODQ Anomie cascabelle Pododesmus cepio Abalone jingle shell Anomiidae BIVALVIA ABX Ormeaux nca Haliotis spp Abalones nei Haliotidae GASTROPODA REN Sébaste rose Sebastes fasciatus Acadian redfish Scorpaenidae SCORPAENIFORMES YNA Acoupa toeroe Cynoscion acoupa Acoupa weakfish Sciaenidae PERCOIDEI HSV Pourpre aiguillonnee Thais aculeata Aculeate rock shell Muricidae GASTROPODA GBQ Troque d'Adanson Gibbula adansoni Adanson's gibbula Trochidae GASTROPODA NKA Natice d'Adanson Natica adansoni Adanson's moon snail Naticidae GASTROPODA GLW Tagal d'Adanson Tagelus adansonii Adanson's tagelus Solecurtidae BIVALVIA PYD Manchot d'Adélie Pygoscelis adeliae Adelie penguin Spheniscidae SPHENISCIFORMES QFT Maconde aden Synagrops adeni Aden splitfin Acropomatidae PERCOIDEI NIV Crevette adonis Parapenaeopsis venusta Adonis shrimp Penaeidae NATANTIA DJD Modiole adriatique Modiolus adriaticus Adriatic horse mussel Mytilidae BIVALVIA AAA Esturgeon de l'Adriatique Acipenser naccarii Adriatic sturgeon Acipenseridae ACIPENSERIFORMES FCV Fucus d'Adriatique Fucus virsoides Adriatic wrack Fucaceae PHAEOPHYCEAE IRR Mitre brûlée Mitra eremitarum Adusta miter Mitridae GASTROPODA KCE Murex bruni Chicoreus brunneus Adusta murex Muricidae GASTROPODA AES Crevette ésope Pandalus montagui Aesop shrimp Pandalidae NATANTIA CGM Poisson-chat, hybride Clarias gariepinus x C. macrocephalus Africa-bighead catfish, hybrid Clariidae SILURIFORMES SUF Ange de mer africain Squatina africana African angelshark Squatinidae SQUALIFORMES -

I Rozporządzenia
31.3.2009 PL Dziennik Urzędowy Unii Europejskiej L 87/1 I (Akty przyjęte na mocy Traktatów WE/Euratom, których publikacja jest obowiązkowa) ROZPORZĄDZENIA ROZPORZĄDZENIE PARLAMENTU EUROPEJSKIEGO I RADY (WE) NR 216/2009 z dnia 11 marca 2009 r. w sprawie przekazywania przez paDstwa czBonkowskie prowadzące poBowy na okre[lonych obszarach, innych ni| póBnocny Atlantyk, danych statystycznych o poBowach nominalnych (przeksztaBcenie) (Tekst mający znaczenie dla EOG) PARLAMENT EUROPEJSKI I RADA UNII EUROPEJSKIEJ, ryboBówstwa oraz zapewnienie niezbędnej infrastruktury do przetwarzania i kontroli wiarygodno[ci powy|szych uwzględniając Traktat ustanawiający Wspólnotę Europejską, danych jest pierwszym i podstawowym obowiązkiem w szczególno[ci jego art. 285 ust. 1, paDstw czBonkowskich. uwzględniając wniosek Komisji, (5) Kilka paDstw czBonkowskich wystąpiBo z wnioskiem o przedkBadanie danych w innej formie albo za pomocą stanowiąc zgodnie z procedurą okre[loną w art. 251 Traktatu (1), innych [rodków komunikacji ni| okre[lone w załącz- niku V (odpowiednik kwestionariuszy Statlant). a tak|e mając na uwadze, co następuje: (1) Rozporządzenie Rady (WE) nr 2597/95 z dnia 23 paz- (6) Zrodki konieczne do wykonania niniejszego rozporządze- dziernika 1995 r. w sprawie przekazywania przez paDstwa nia powinny zostać przyjęte zgodnie z decyzją Rady 1999/ czBonkowskie prowadzące poBowy na niektórych obsza- 486/WE z dnia 28 czerwca 1999 r., ustanawiającą warunki rach, innych ni| póBnocny Atlantyk, danych statystycznych wykonywania uprawnieD wykonawczych przyznanych dotyczących poBowów nominalnych (2) zostaBo kilkakrotnie Komisji (4). znacząco zmienione (3). Poniewa| mają być do niego wprowadzone kolejne zmiany, nale|y je przeredagować w celu zapewnienia jasno[ci. (7) W szczególno[ci nale|y przyznać Komisji uprawienienie do dostosowywania wykazów statystycznych obszarów poBo- (2) Wspólnota Europejska uzyskaBa czBonkostwo w Organizacji wowych lub ich podrejonów i gatunków. -

Tetrapturus Pfluegeri Robins & De Sylva, 1963 Thunnus Alalunga
Centros Grupo de Classe Ordem Família Nome científico avaliação Peixes Marinhos TAMAR Actinopterygii Scombriformes Ariommatidae Ariomma melanum (Ginsburg, 1954) Peixes Marinhos TAMAR Actinopterygii Scombriformes Ariommatidae Ariomma bondi Fowler, 1930 Peixes Marinhos TAMAR Actinopterygii Scombriformes Centrolophidae Seriolella porosa Guichenot, 1848 Peixes Marinhos TAMAR Actinopterygii Scombriformes Centrolophidae Hyperoglyphe macrophthalma (Miranda Ribeiro, 1915) Peixes Marinhos TAMAR Actinopterygii Scombriformes Centrolophidae Centrolophus niger (Gmelin, 1789) Peixes Marinhos TAMAR Actinopterygii Carangiformes Coryphaenidae Coryphaena hippurus Linnaeus, 1758 Peixes Marinhos TAMAR Actinopterygii Carangiformes Coryphaenidae Coryphaena equiselis Linnaeus, 1758 Peixes Marinhos TAMAR Actinopterygii Scombriformes Gempylidae Thyrsitops lepidopoides (Cuvier, 1832) Peixes Marinhos TAMAR Actinopterygii Scombriformes Gempylidae Ruvettus pretiosus Cocco, 1883 Peixes Marinhos TAMAR Actinopterygii Scombriformes Gempylidae Promethichthys prometheus (Cuvier, 1832) Peixes Marinhos TAMAR Actinopterygii Scombriformes Gempylidae Nesiarchus nasutus Johnson, 1865 Peixes Marinhos TAMAR Actinopterygii Scombriformes Gempylidae Neoepinnula americana (Grey, 1953) Peixes Marinhos TAMAR Actinopterygii Scombriformes Gempylidae Nealotus tripes Johnson, 1865 Peixes Marinhos TAMAR Actinopterygii Scombriformes Gempylidae Lepidocybium flavobrunneum (Smith, 1843) Peixes Marinhos TAMAR Actinopterygii Scombriformes Gempylidae Gempylus serpens Cuvier, 1829 Peixes Marinhos