Goals and Scope of the Technical Consultation Meeting Katri Tala
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Drug & Alcohol Testing Program
Pottawattmie County Drug & Alcohol Testing Program Appendix A Table of Contents POLICY STATEMENT ...................................................................................................................................... 3 SCOPE ............................................................................................................................................................ 4 EDUCATION AND TRAINING .......................................................................................................................... 4 DESIGNATED EMPLOYER REPRESENTATIVE (DER): ....................................................................................... 5 DUTY TO COOPERATE ................................................................................................................................... 5 EMPLOYEE ADMISSION OF ALCOHOL AND CONTROLLED SUBSTANCE USE: (49 CFR Part 382.121) ... 6 PROHIBITED DRUGS AND ILLEGALLY USED CONTROLLED SUBSTANCES: ..................................................... 7 PROHIBITED BEHAVIOR AND CONDUCT: ...................................................................................................... 8 DRUG & ALCOHOL TESTING REQUIREMENTS (49 CFR, Part 40 & 382) ............................................... 10 DRUG & ALCOHOL TESTING CIRCUMSTANCES (49 CFR Part 40 & 382) .............................................. 12 A. Pre-Employment Testing: .................................................................................................... 12 B. Reasonable Suspicion Testing: ......................................................................................... -

Guidelines for the Forensic Analysis of Drugs Facilitating Sexual Assault and Other Criminal Acts
Vienna International Centre, PO Box 500, 1400 Vienna, Austria Tel.: (+43-1) 26060-0, Fax: (+43-1) 26060-5866, www.unodc.org Guidelines for the Forensic analysis of drugs facilitating sexual assault and other criminal acts United Nations publication Printed in Austria ST/NAR/45 *1186331*V.11-86331—December 2011 —300 Photo credits: UNODC Photo Library, iStock.com/Abel Mitja Varela Laboratory and Scientific Section UNITED NATIONS OFFICE ON DRUGS AND CRIME Vienna Guidelines for the forensic analysis of drugs facilitating sexual assault and other criminal acts UNITED NATIONS New York, 2011 ST/NAR/45 © United Nations, December 2011. All rights reserved. The designations employed and the presentation of material in this publication do not imply the expression of any opinion whatsoever on the part of the Secretariat of the United Nations concerning the legal status of any country, territory, city or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. This publication has not been formally edited. Publishing production: English, Publishing and Library Section, United Nations Office at Vienna. List of abbreviations . v Acknowledgements .......................................... vii 1. Introduction............................................. 1 1.1. Background ........................................ 1 1.2. Purpose and scope of the manual ...................... 2 2. Investigative and analytical challenges ....................... 5 3 Evidence collection ...................................... 9 3.1. Evidence collection kits .............................. 9 3.2. Sample transfer and storage........................... 10 3.3. Biological samples and sampling ...................... 11 3.4. Other samples ...................................... 12 4. Analytical considerations .................................. 13 4.1. Substances encountered in DFSA and other DFC cases .... 13 4.2. Procedures and analytical strategy...................... 14 4.3. Analytical methodology .............................. 15 4.4. -

Metabolic-Hydroxy and Carboxy Functionalization of Alkyl Moieties in Drug Molecules: Prediction of Structure Influence and Pharmacologic Activity
molecules Review Metabolic-Hydroxy and Carboxy Functionalization of Alkyl Moieties in Drug Molecules: Prediction of Structure Influence and Pharmacologic Activity Babiker M. El-Haj 1,* and Samrein B.M. Ahmed 2 1 Department of Pharmaceutical Sciences, College of Pharmacy and Health Sciences, University of Science and Technology of Fujairah, Fufairah 00971, UAE 2 College of Medicine, Sharjah Institute for Medical Research, University of Sharjah, Sharjah 00971, UAE; [email protected] * Correspondence: [email protected] Received: 6 February 2020; Accepted: 7 April 2020; Published: 22 April 2020 Abstract: Alkyl moieties—open chain or cyclic, linear, or branched—are common in drug molecules. The hydrophobicity of alkyl moieties in drug molecules is modified by metabolic hydroxy functionalization via free-radical intermediates to give primary, secondary, or tertiary alcohols depending on the class of the substrate carbon. The hydroxymethyl groups resulting from the functionalization of methyl groups are mostly oxidized further to carboxyl groups to give carboxy metabolites. As observed from the surveyed cases in this review, hydroxy functionalization leads to loss, attenuation, or retention of pharmacologic activity with respect to the parent drug. On the other hand, carboxy functionalization leads to a loss of activity with the exception of only a few cases in which activity is retained. The exceptions are those groups in which the carboxy functionalization occurs at a position distant from a well-defined primary pharmacophore. Some hydroxy metabolites, which are equiactive with their parent drugs, have been developed into ester prodrugs while carboxy metabolites, which are equiactive to their parent drugs, have been developed into drugs as per se. -

Chronic Use of Opioid Medications Before and After Bariatric Surgery
Supplementary Online Content Raebel MA, Newcomer SR, Reifler LM, et al. Chronic use of opioid medications before and after bariatric surgery. JAMA. doi:10.1001/jama.2013.278344 eFigure. Opioid Dispensings in 60 Days Before and 60 Days After Bariatric Surgery eTable 1. Opioid Classification and Morphine Equivalents Conversion Factors for Opioids Administered by Tablet, Capsule, Liquid, Transdermal Patch, and Transmucosal Formulations eTable 2. ICD-9 Codes for Comorbid Diagnosis of Substance Abuse, Anxiety, Posttraumatic Stress Disorder, and/or Depression eTable 3. ICD-9 Codes for Inclusion and Exclusion of Chronic Pain Diagnoses eTable 4. Therapeutic Classes, Generic Names, and Selected Brand Names of Covariate Medications eTable 5. Types of Opioids Dispensed During the Year Before and the Year After Bariatric Surgery Among Presurgery Chronic Opioid Users eTable 6. Types of Opioids Used Before and After Bariatric Surgery Among 933 Individuals With Chronic Opioid Use Before Bariatric Surgery eTable 7. Unadjusted Characteristics of Chronic Opioid Users According to Whether or Not Both Presurgery and Postsurgery Body Mass Indexes (BMIs) Data Were Available eTable 8. Presurgery and Postsurgery Use of Opioids and Selected Other Analgesic and Adjunctive Pain Medication Classes Among Presurgery Chronic Opioid Users With Presurgery Chronic Pain Diagnoses This supplementary material has been provided by the authors to give readers additional information about their work. © 2013 American Medical Association. All rights reserved. Downloaded From: -

The In¯Uence of Medication on Erectile Function
International Journal of Impotence Research (1997) 9, 17±26 ß 1997 Stockton Press All rights reserved 0955-9930/97 $12.00 The in¯uence of medication on erectile function W Meinhardt1, RF Kropman2, P Vermeij3, AAB Lycklama aÁ Nijeholt4 and J Zwartendijk4 1Department of Urology, Netherlands Cancer Institute/Antoni van Leeuwenhoek Hospital, Plesmanlaan 121, 1066 CX Amsterdam, The Netherlands; 2Department of Urology, Leyenburg Hospital, Leyweg 275, 2545 CH The Hague, The Netherlands; 3Pharmacy; and 4Department of Urology, Leiden University Hospital, P.O. Box 9600, 2300 RC Leiden, The Netherlands Keywords: impotence; side-effect; antipsychotic; antihypertensive; physiology; erectile function Introduction stopped their antihypertensive treatment over a ®ve year period, because of side-effects on sexual function.5 In the drug registration procedures sexual Several physiological mechanisms are involved in function is not a major issue. This means that erectile function. A negative in¯uence of prescrip- knowledge of the problem is mainly dependent on tion-drugs on these mechanisms will not always case reports and the lists from side effect registries.6±8 come to the attention of the clinician, whereas a Another way of looking at the problem is drug causing priapism will rarely escape the atten- combining available data on mechanisms of action tion. of drugs with the knowledge of the physiological When erectile function is in¯uenced in a negative mechanisms involved in erectile function. The way compensation may occur. For example, age- advantage of this approach is that remedies may related penile sensory disorders may be compen- evolve from it. sated for by extra stimulation.1 Diminished in¯ux of In this paper we will discuss the subject in the blood will lead to a slower onset of the erection, but following order: may be accepted. -

Designer Drugs SUBJECT FORENSIC SCIENCE
SUBJECT FORENSIC SCIENCE Paper No. and Title PAPER No. 9: Drugs of Abuse Module No. and Title MODULE No. 17: Designer Drugs Module Tag FSC_P9_M17 FORENSIC SCIENCE PAPER No. 9: Drugs of Abuse MODULE No. 17: Designer Drugs TABLE OF CONTENTS 1. Learning Outcomes 2. Introduction 3. Forensic Issues 4. Classification of Designer Drugs 5. Some Notable Designer Drugs 6. Forensic Analysis of Designer Drugs 7. Summary FORENSIC SCIENCE PAPER No. 9: Drugs of Abuse MODULE No. 17: Designer Drugs 1. Learning Outcomes After studying this module, you shall be able to know about: The significance of Designer Drugs Classification of Designer Drugs Forensic analysis of Designer Drugs 2. Introduction Over ages, humans fortunately discovered that certain ingested plants were a source of unique satisfying feelings, beyond satiety. Some were mildly affecting (e.g. nicotine, caffeine), others enhanced mood or altered perception, reduced pain, intoxicated, or produced euphoria (e.g. alcohol, marijuana, hallucinogens, opiates, cocaine). In the past two centuries, consumption of these psychoactive substances expanded rapidly. Decontamination of the active chemicals, distribution by manoeuvres for maximum effect and global marketing contributed to this expansion. Modern chemistry has produced a huge range of variations of these plant products, paralleled by an unprecedented level of adverse biological, behavioural, medical and social consequences. Following this phenomenon, Designer drugs are produced to be similar to, but not identical with Psychoactive Drugs that are illegal to possess or sell for human consumption, unless for medical purposes. A recurring threat to public health, the Designer Drug sub-culture has burst out over the past decade. -

BUTALBITAL ANALGESICS Allzital (Butalbital-Acetaminophen
BUTALBITAL ANALGESICS Allzital (butalbital-acetaminophen), butalbital-aspirin-caffeine, butalbital-aspirin-caffeine- codeine, butalbital-acetaminophen, butalbital-acetaminophen-caffeine, butalbital- acetaminophen-caffeine-codeine, Vanatol LQ (butalbital-acetaminophen-caffeine liquid oral solution) RATIONALE FOR INCLUSION IN PA PROGRAM Background Butalbital containing products are non-opioid analgesics that contain a combination of different drug products indicated for the relief of the symptom complex of tension (or muscle contraction) headache pain. Butalbital is a short to intermediate-acting barbiturate that works in concert with acetaminophen, an antipyretic non-salicylate agent, aspirin, a pain-relieving NSAID, and caffeine, a stimulant that works in the CNS, to decrease pain via a mechanism that isn’t well understood. Butalbital is a habit-forming drug that potentiates the effects of other commonly abuse drugs or substances like alcohol. Caffeine might help increase vasodilation and smooth muscle relaxation, while butalbital is thought to help balance the CNS stimulation caused by caffeine and produces depressant effects (1). Regulatory Status FDA approved indication: Butalbital containing products are used in the relief of the symptom complex of tension or muscle contraction headaches (2-8). Frequent use of acute medications is generally thought to cause medication-overuse headache. To decrease the risk of medication-overuse headache (“rebound headache” or “drug-induced headache”) many experts limit acute therapy to two headache days per week on a regular basis. Based on concerns of overuse, medication-overuse headache, and withdrawal, the use of butalbital-containing analgesics should be limited and carefully monitored. The Allzital limit is set to the maximum of 12 doses per day for acute treatment of 8 headaches per month as this product contains less butalbital than other products. -

Definition of Controlled Substance Schedules
UPDATED MARCH 2018 DEFINITION OF CONTROLLED SUBSTANCE SCHEDULES Drugs and other substances that are considered controlled substances under the Controlled Substances Act (CSA) are divided into five schedules. An updated and complete list of the schedules is published annually in Title 21 Code of Federal Regulations (C.F.R.) §§ 1308.11 through 1308.15. Substances are placed in their respective schedules based on whether they have a currently accepted medical use in treatment in the U.S., their relative abuse potential, and likelihood of causing dependence when abused. Examples of the drugs in each schedule are listed below. Schedule I Controlled Substances Substances in this schedule have no currently accepted medical use in the United States, a lack of accepted safety for use under medical supervision, and a high potential for abuse. Some examples of substances listed in Schedule I are: heroin, lysergic acid diethylamide (LSD), marijuana (cannabis), peyote, methaqualone, and 3,4- methylenedioxymethamphetamine ("Ecstasy"). Schedule II/IIN Controlled Substances (2/2N) Substances in this schedule have a high potential for abuse which may lead to severe psychological or physical dependence. Examples of Schedule II narcotics include: hydromorphone (Dilaudid®), methadone (Dolophine®), meperidine (Demerol®), oxycodone (OxyContin®, Percocet®), and fentanyl (Sublimaze®, Duragesic®). Other Schedule II narcotics include: morphine, opium, codeine, and hydrocodone. Examples of Schedule IIN stimulants include: amphetamine (Dexedrine®, Adderall®), methamphetamine (Desoxyn®), and methylphenidate (Ritalin®). Other Schedule II substances include: amobarbital, glutethimide, and pentobarbital. 1 Schedule III/IIIN Controlled Substances (3/3N) Substances in this schedule have a potential for abuse less than substances in Schedules I or II and abuse may lead to moderate or low physical dependence or high psychological dependence. -
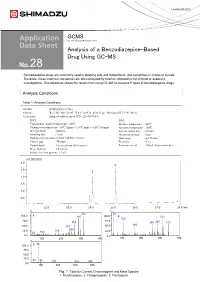
Analysis of a Benzodiazepine-Based Drug Using GC-MS 28
LAAN-E-MS-E028 GCMS Gas Chromatograph Mass Spectrometer Analysis of a Benzodiazepine-Based Drug Using GC-MS 28 Benzodiazepine drugs are commonly used in sleeping aids and tranquilizers, and sometimes in crimes or suicide. Therefore, these chemical substances are often analyzed by forensic laboratories for criminal or academic investigations. This datasheet shows the results from using GC-MS to measure 9 types of benzodiazepine drugs. Analysis Conditions Table 1: Analysis Conditions GC-MS :GCMS-QP2010 Ultra Column : Rxi®-5Sil MS (30 mL. X 0.25 mmI.D., df=0.25 µm, Shimadzu GLC P/N:13623) Glass insert :Silanized splitless insert (P/N: 221-48876-03) [GC] [MS] Vaporization chamber temperature : 260℃ Interface temperature : 280℃ Column oven temperature : 60℃ (2min) -> (10℃/min) -> 320℃ (10min) Ion source temperature : 200℃ Injection mode : Splitless Solvent elution time : 2.0 min Sampling time : 1 min Measurement mode : Scan High pressure injection method: 250 kPa (1.5 min) Mass range : m/z 35-600 Carrier gas : Helium Event time : 0.3 sec Control mode :Linear velocity (45.6 cm/sec) Emission current : 150 µA (high sensitivity) Purge flow rate :3.0 ml/min Sample injection quantity :1.0 µL (x1,000,000) 3.0 3 2.5 2 2.0 1.5 1.0 1 0.5 22.0 23.0 24.0 25.0 26.0 27.0 28.0 min % % 312 55 100.0 1 285 100.0 2 313 109 75.0 75.0 266 259287 342 166 50.0 238 50.0 248 25.0 183 25.0 63 109 0.0 0.0 100 200 300 400 100 200 300 400 % 86 100.0 3 75.0 50.0 25.0 58 99 183 315 387 0.0 100 200 300 400 Fig. -

Proposed Regulation of the State Board of Pharmacy
PROPOSED REGULATION OF THE STATE BOARD OF PHARMACY LCB File No. R133-14 Workshop July 24, 2014 NAC 453.540 Schedule IV. (NRS 453.146, 639.070) 1. Schedule IV consists of the drugs and other substances listed in this section, by whatever official, common, usual, chemical or trade name designated. 2. Unless specifically excepted or unless listed in another schedule, any material, compound, mixture or preparation containing any of the following narcotic drugs, including, without limitation, their salts, calculated as the free anhydrous base of alkaloid, is hereby enumerated on schedule IV, in quantities: (a) Not more than 1 milligram of difenoxin and not less than 25 micrograms of atropine sulfate per dosage unit; or (b) Dextropropoxyphene (alpha-(+)-4-dimethylamino-1,2-diphenyl-3-methyl-2-propionoxy- butane). 3. Unless specifically excepted or unless listed in another schedule, any material, compound, mixture or preparation which contains any quantity of the following substances, including, without limitation, their salts, isomers and salts of isomers, is hereby enumerated on schedule IV, whenever the existence of such salts, isomers and salts of isomers is possible within the specific chemical designation: Alprazolam; Barbital; Bromazepam; Butorphanol; Camazepam; Carisoprodol; Chloral betaine; Chloral hydrate; Chlordiazepoxide; Clobazam; Clonazepam; Clorazepate; Clotiazepam; Cloxazolam; Delorazepam; Diazepam; Dichloralphenazone; Estazolam; Ethchlorvynol; Ethyl loflazepate; Fludiazepam; Flunitrazepam; --1-- Agency Draft of Proposed Regulation R133-14 Flurazepam; Halazepam; Haloxazolam; Ketazolam; Loprazolam; Lorazepam; Lormetazepam; Mebutamate; Medazepam; Meprobamate; Methohexital; Methylphenobarbital (mephobarbital); Midazolam; Nimetazepam; Nitrazepam; Nordiazepam; Oxazepam; Oxazolam; Paraldehyde; Petrichloral; Phenobarbital; Pinazepam; Prazepam; Quazepam; Tramadol (2-((dimethylamino)methyl)-1-(3-methoxyphenyl)cyclohexanol) Temazepam; Tetrazepam; Triazolam; Zaleplon; Zolpidem; or Zopiclone. -

Recommended Methods for the Identification and Analysis of Fentanyl and Its Analogues in Biological Specimens
Recommended methods for the Identification and Analysis of Fentanyl and its Analogues in Biological Specimens MANUAL FOR USE BY NATIONAL DRUG ANALYSIS LABORATORIES Laboratory and Scientific Section UNITED NATIONS OFFICE ON DRUGS AND CRIME Vienna Recommended Methods for the Identification and Analysis of Fentanyl and its Analogues in Biological Specimens MANUAL FOR USE BY NATIONAL DRUG ANALYSIS LABORATORIES UNITED NATIONS Vienna, 2017 Note Operating and experimental conditions are reproduced from the original reference materials, including unpublished methods, validated and used in selected national laboratories as per the list of references. A number of alternative conditions and substitution of named commercial products may provide comparable results in many cases. However, any modification has to be validated before it is integrated into laboratory routines. ST/NAR/53 Original language: English © United Nations, November 2017. All rights reserved. The designations employed and the presentation of material in this publication do not imply the expression of any opinion whatsoever on the part of the Secretariat of the United Nations concerning the legal status of any country, territory, city or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. Mention of names of firms and commercial products does not imply the endorse- ment of the United Nations. This publication has not been formally edited. Publishing production: English, Publishing and Library Section, United Nations Office at Vienna. Acknowledgements The Laboratory and Scientific Section of the UNODC (LSS, headed by Dr. Justice Tettey) wishes to express its appreciation and thanks to Dr. Barry Logan, Center for Forensic Science Research and Education, at the Fredric Rieders Family Founda- tion and NMS Labs, United States; Amanda L.A. -

WO 2012/148799 Al 1 November 2012 (01.11.2012) P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2012/148799 Al 1 November 2012 (01.11.2012) P O P C T (51) International Patent Classification: (81) Designated States (unless otherwise indicated, for every A61K 9/107 (2006.01) A61K 9/00 (2006.01) kind of national protection available): AE, AG, AL, AM, A 61 47/10 (2006.0V) AO, AT, AU, AZ, BA, BB, BG, BH, BR, BW, BY, BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, DO, (21) International Application Number: DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, HN, PCT/US2012/034361 HR, HU, ID, IL, IN, IS, JP, KE, KG, KM, KN, KP, KR, (22) International Filing Date: KZ, LA, LC, LK, LR, LS, LT, LU, LY, MA, MD, ME, 20 April 2012 (20.04.2012) MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, OM, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SC, SD, (25) Filing Language: English SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, TR, (26) Publication Language: English TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. (30) Priority Data: (84) Designated States (unless otherwise indicated, for every 61/480,259 28 April 201 1 (28.04.201 1) US kind of regional protection available): ARIPO (BW, GH, GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, SZ, TZ, (71) Applicant (for all designated States except US): BOARD UG, ZM, ZW), Eurasian (AM, AZ, BY, KG, KZ, MD, RU, OF REGENTS, THE UNIVERSITY OF TEXAS SYS¬ TJ, TM), European (AL, AT, BE, BG, CH, CY, CZ, DE, TEM [US/US]; 201 West 7th St., Austin, TX 78701 (US).