Designer Drugs SUBJECT FORENSIC SCIENCE
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

2015 Harmonized Tariff Schedule of the United States Chapter 29
Harmonized Tariff Schedule of the United States (2015) Annotated for Statistical Reporting Purposes CHAPTER 29 ORGANIC CHEMICALS VI 29-1 Notes 1. Except where the context otherwise requires, the headings of this chapter apply only to: (a) Separate chemically defined organic compounds, whether or not containing impurities; (b) Mixtures of two or more isomers of the same organic compound (whether or not containing impurities), except mixtures of acyclic hydrocarbon isomers (other than stereoisomers), whether or not saturated (chapter 27); (c) The products of headings 2936 to 2939 or the sugar ethers, sugar acetals and sugar esters, and their salts, of heading 2940, or the products of heading 2941, whether or not chemically defined; (d) Products mentioned in (a), (b) or (c) above dissolved in water; (e) Products mentioned in (a), (b) or (c) above dissolved in other solvents provided that the solution constitutes a normal and necessary method of putting up these products adopted solely for reasons of safety or for transport and that the solvent does not render the product particularly suitable for specific use rather than for general use; (f) The products mentioned in (a), (b), (c), (d) or (e) above with an added stabilizer (including an anticaking agent) necessary for their preservation or transport; (g) The products mentioned in (a), (b), (c), (d), (e) or (f) above with an added antidusting agent or a coloring or odoriferous substance added to facilitate their identification or for safety reasons, provided that the additions do not render the product particularly suitable for specific use rather than for general use; (h) The following products, diluted to standard strengths, for the production of azo dyes: diazonium salts, couplers used for these salts and diazotizable amines and their salts. -

Introduced B.,Byhansen, 16
LB301 LB301 2021 2021 LEGISLATURE OF NEBRASKA ONE HUNDRED SEVENTH LEGISLATURE FIRST SESSION LEGISLATIVE BILL 301 Introduced by Hansen, B., 16. Read first time January 12, 2021 Committee: Judiciary 1 A BILL FOR AN ACT relating to the Uniform Controlled Substances Act; to 2 amend sections 28-401, 28-405, and 28-416, Revised Statutes 3 Cumulative Supplement, 2020; to redefine terms; to change drug 4 schedules and adopt federal drug provisions; to change a penalty 5 provision; and to repeal the original sections. 6 Be it enacted by the people of the State of Nebraska, -1- LB301 LB301 2021 2021 1 Section 1. Section 28-401, Revised Statutes Cumulative Supplement, 2 2020, is amended to read: 3 28-401 As used in the Uniform Controlled Substances Act, unless the 4 context otherwise requires: 5 (1) Administer means to directly apply a controlled substance by 6 injection, inhalation, ingestion, or any other means to the body of a 7 patient or research subject; 8 (2) Agent means an authorized person who acts on behalf of or at the 9 direction of another person but does not include a common or contract 10 carrier, public warehouse keeper, or employee of a carrier or warehouse 11 keeper; 12 (3) Administration means the Drug Enforcement Administration of the 13 United States Department of Justice; 14 (4) Controlled substance means a drug, biological, substance, or 15 immediate precursor in Schedules I through V of section 28-405. 16 Controlled substance does not include distilled spirits, wine, malt 17 beverages, tobacco, hemp, or any nonnarcotic substance if such substance 18 may, under the Federal Food, Drug, and Cosmetic Act, 21 U.S.C. -

House Bill No.11 (2019)
LEGISLATURE OF THE STATE OF IDAHO Sixty-fifth Legislature First Regular Session - 2019 IN THE HOUSE OF REPRESENTATIVES HOUSE BILL NO. 11 BY HEALTH AND WELFARE COMMITTEE 1 AN ACT 2 RELATING TO CONTROLLED SUBSTANCES; AMENDING SECTION 37-2705, IDAHO CODE, TO 3 REVISE THE LIST OF SCHEDULE I CONTROLLED SUBSTANCES; AMENDING SECTION 4 37-2709, IDAHO CODE, TO PROVIDE AN EXCLUSION AND TO MAKE A TECHNICAL 5 CORRECTION; AMENDING SECTION 37-2713, IDAHO CODE, TO REVISE THE LIST OF 6 SCHEDULE V DRUGS AND SUBSTANCES; AND DECLARING AN EMERGENCY. 7 Be It Enacted by the Legislature of the State of Idaho: 8 SECTION 1. That Section 37-2705, Idaho Code, be, and the same is hereby 9 amended to read as follows: 10 37-2705. SCHEDULE I. (a) The controlled substances listed in this sec- 11 tion are included in schedule I. 12 (b) Any of the following opiates, including their isomers, esters, 13 ethers, salts, and salts of isomers, esters, and ethers, unless specifically 14 excepted, whenever the existence of these isomers, esters, ethers and salts 15 is possible within the specific chemical designation: 16 (1) Acetyl-alpha-methylfentanyl (N-[1-(1-methyl-2-phenethyl)-4-pip- 17 eridinyl]-N-phenylacetamide); 18 (2) Acetylmethadol; 19 (3) Acetyl fentanyl (N-(1-phenethylpiperidin-4-yl)-N-phenylac- 20 etamide); 21 (4) Allylprodine; 22 (5) Alphacetylmethadol (except levo-alphacetylmethadol also known as 23 levo-alpha-acetylmethadol, levomethadyl acetate or LAAM); 24 (6) Alphameprodine; 25 (7) Alphamethadol; 26 (8) Alpha-methylfentanyl; 27 (9) Alpha-methylthiofentanyl -

Tennessee Drug Statutes (Listed in Numerical Order)
Tennessee Drug Statutes (listed in numerical order) 39-17-405. Criteria for Schedule I. • The commissioner of mental health and substance abuse services, upon the agreement of the commissioner of health, shall place a substance in Schedule I upon finding that the substance has: o (1) High potential for abuse; and o (2) No accepted medical use in treatment in the United States or lacks accepted safety for use in treatment under medical supervision. 39-17-406. Controlled substances in Schedule I. • (a) Schedule I consists of the drugs and other substances, by whatever official name, common or usual name, chemical name, or brand name designated, listed in this section. • (b) Opiates, unless specifically excepted or unless listed in another schedule, means any of the following opiates, including their isomers, esters, ethers, salts and salts of isomers, esters, and ethers, whenever the existence of such isomers, esters, ethers, and salts is possible within the specific chemical designation. For the purposes of subdivision (b)(34) only, the term isomer includes the optical and geometric isomers. o (1) Acetyl-alpha-methylfentanyl (N-[1-(1-methyl-2-phenethyl)-4- piperidinyl]-N-phenylacetamide); o (2) Acetylmethadol; o (3) Allylprodine; o (4) Alphacetylmethadol (except levo-alphacetylmethadol also known as levo-alpha-acetylmethadol; levomethadyl acetate; or LAAM); o (5) Alphameprodine; o (6) Alphamethadol; o (7) Alpha-methylfentanyl (N-[1-(alpha-methyl-beta-phenyl)ethyl-4- piperidyl]propionanilide; 1-(1-methyl-2-phenylethyl)-4-(N- propanilido)piperidine; -

Alcohol and Drug Abuse Subchapter 9 Regulated Drug Rule 1.0 Authority
Chapter 8 – Alcohol and Drug Abuse Subchapter 9 Regulated Drug Rule 1.0 Authority This rule is established under the authority of 18 V.S.A. §§ 4201 and 4202 which authorizes the Vermont Board of Health to designate regulated drugs for the protection of public health and safety. 2.0 Purpose This rule designates drugs and other chemical substances that are illegal or judged to be potentially fatal or harmful for human consumption unless prescribed and dispensed by a professional licensed to prescribe or dispense them, and used in accordance with the prescription. The rule restricts the possession of certain drugs above a specified quantity. The rule also establishes benchmark unlawful dosages for certain drugs to provide a baseline for use by prosecutors to seek enhanced penalties for possession of higher quantities of the drug in accordance with multipliers found at 18 V.S.A. § 4234. 3.0 Definitions 3.1 “Analog” means one of a group of chemical components similar in structure but different with respect to elemental composition. It can differ in one or more atoms, functional groups or substructures, which are replaced with other atoms, groups or substructures. 3.2 “Benchmark Unlawful Dosage” means the quantity of a drug commonly consumed over a twenty-four hour period for any therapeutic purpose, as established by the manufacturer of the drug. Benchmark Unlawful dosage is not a medical or pharmacologic concept with any implication for medical practice. Instead, it is a legal concept established only for the purpose of calculating penalties for improper sale, possession, or dispensing of drugs pursuant to 18 V.S.A. -

CDL Drug and Alcohol Testing
CDL Drug and Alcohol Program Department of Environmental Health & Safety Phone: (410) 704-2949 Fax: (410) 704-2993 Emergency: (410) 704-2133 Email: [email protected] Website: www.towson.edu/ehs/index.html REVISED December 2013 Commercial Driver License Drug and Alcohol Program TU Drug and Alcohol Policy and Testing Procedures Applicable to Employees Required by Job Function to have a Commercial Driver’s License I. Policy Towson University recognizes the safety-sensitive function of its employees who are required by their job function to hold a commercial driver’s license (CDL). As an employer, the University has a responsibility to help prevent accidents and injuries resulting from the misuse of alcohol and use of controlled substances1 by employees who drive commercial motor vehicles. In order to ensure the safety of employees with CDL licenses (CDL Employee(s)) and the campus community, and to comply with the Omnibus Transportation Employee Testing Act of 1991 (the Act), the University adopts as policy the prohibitions against the misuse of alcohol and the use of controlled substances by CDL Employees and the drug and alcohol testing procedures as set forth in the federal regulations implementing the Act.2 Copies of these federal regulations are available on the Department of Environmental Health and Safety’s website (www.towson.edu/ehs/index.html) or by calling the department at x4-2949. II. University Contact Regarding Policy and Procedures Questions about these policies and procedures should be addressed to the Department of Environmental Health and Safety (x4-2949) or the Director of Human Resources (x4-4053). -

METHAQUALONE Latest Revision: February 15, 1999
METHAQUALONE Latest Revision: February 15, 1999 1. SYNONYMS CFR: Methaqualone CAS #: Base: 72-44-6 Hydrochloride: 340-56-7 Other Names: 2-Methyl-3-(2-methylphenyl)-4-(3H)-quinazolinone 2-Methyl-3-o-tolyl-4-(3H)-quinazolinone Dormogen Metolquizolone Mandrax MAOA Motolon Quaalude Sopor 2. CHEMICAL AND PHYSICAL DATA 2.1. CHEMICAL DATA Form Chemical Formula Molecular Weight Melting Point (°C) Base C16H14N2O 250.3 114-116 Hydrochloride C16H15N2OCl 286.8 255-265 2.2. SOLUBILITY Form A C E H M W Base *** S P *** PS I Hydrochloride S S I *** S PS A = acetone, C = chloroform, E = ether, H = hexane, M = methanol and W = water, VS = very soluble, FS = freely soluble, S = soluble, PS = sparingly soluble, SS = slightly soluble, VSS = very slightly soluble and I = insoluble 3. SCREENING TECHNIQUES 3.1. COLOR TESTS REAGENT COLOR PRODUCED Acidified cobalt thiocyanate Blue Fischer-Morris Pink 3.2. CRYSTAL TESTS REAGENT CRYSTALS FORMED Potassium permanganate solution Plates Sodium carbonate solution Needles 3.3. THIN-LAYER CHROMATOGRAPHY Visualization Acidified iodoplatinate solution Brown-violet Dragendorff spray Yellow-orange RELATIVE R1 COMPOUND System System System TLC6 TLC5 TLC7 mecloqualone 0.8 1.0 1.0 methaqualone 1.0 1.0 1.0 o-toluidine 1.3 1.6 1.6 3.4. GAS CHROMATOGRAPHY Methaqualone is a thermally stable compound well suited for gas chromatography. It may however, have a similar retention time with cocaine in certain temperature programs. Method MEQ-GCS1 Instrument: Gas chromatograph operated in split mode with FID Column: 5% phenyl/95% methyl silicone 12 m x 0.2 mm x 0.33 µm film thickness Carrier gas: Helium at 1.0 mL/min Temperatures: Injector: 270°C Detector: 280°C Oven program: 1) 175°C initial temperature for 1.0 min 2) Ramp to 275°C at 15°C/min 3) Hold final temperature for 3.0 min Injection Parameters: Split Ratio = 60:1, 1 µL injected Samples are to be dissolved in chloroform and filtered. -

Laws 2021, LB236, § 4
LB236 LB236 2021 2021 LEGISLATIVE BILL 236 Approved by the Governor May 26, 2021 Introduced by Brewer, 43; Clements, 2; Erdman, 47; Slama, 1; Lindstrom, 18; Murman, 38; Halloran, 33; Hansen, B., 16; McDonnell, 5; Briese, 41; Lowe, 37; Groene, 42; Sanders, 45; Bostelman, 23; Albrecht, 17; Dorn, 30; Linehan, 39; Friesen, 34; Aguilar, 35; Gragert, 40; Kolterman, 24; Williams, 36; Brandt, 32. A BILL FOR AN ACT relating to law; to amend sections 28-1202 and 69-2436, Reissue Revised Statutes of Nebraska, and sections 28-401 and 28-405, Revised Statutes Cumulative Supplement, 2020; to redefine terms, change drug schedules, and adopt federal drug provisions under the Uniform Controlled Substances Act; to provide an exception to the offense of carrying a concealed weapon as prescribed; to define a term; to change provisions relating to renewal of a permit to carry a concealed handgun; to provide a duty for the Nebraska State Patrol; to eliminate an obsolete provision; to harmonize provisions; and to repeal the original sections. Be it enacted by the people of the State of Nebraska, Section 1. Section 28-401, Revised Statutes Cumulative Supplement, 2020, is amended to read: 28-401 As used in the Uniform Controlled Substances Act, unless the context otherwise requires: (1) Administer means to directly apply a controlled substance by injection, inhalation, ingestion, or any other means to the body of a patient or research subject; (2) Agent means an authorized person who acts on behalf of or at the direction of another person but does not include a common or contract carrier, public warehouse keeper, or employee of a carrier or warehouse keeper; (3) Administration means the Drug Enforcement Administration of the United States Department of Justice; (4) Controlled substance means a drug, biological, substance, or immediate precursor in Schedules I through V of section 28-405. -

Panacea Or Pipe Dream: Does Portugal's Drug Decriminalization Policy Translate for Hawaii? Honolulu, HI: Legislative Reference Bureau, January 2017
PANACEA OR PIPE DREAM: DOES PORTUGAL’S DRUG DECRIMINALIZATION POLICY TRANSLATE FOR HAWAII? Primary Researchers: PAUL KANOHO JOHNNY BRANNON MATTHEW PRELLBERG Research Attorneys Supervising Researcher: EDWIN L. BAKER Senior Researcher Report No. 1, 2017 Legislative Reference Bureau State Capitol Honolulu, Hawaii http://lrbhawaii.org This report has been cataloged as follows: Kanoho, Paul + Brannon, Johnny + Prellberg, Matthew + Baker, Edwin L. Panacea or pipe dream: does Portugal's drug decriminalization policy translate for Hawaii? Honolulu, HI: Legislative Reference Bureau, January 2017. 1. Drug legalization – Portugal. 2. Drug legalization – Hawaii. KFH421.5 L35 A25 17-1 FOREWORD This report was prepared in response to House Concurrent Resolution No. 127, H.D. 1, S.D. 1 (2016), which requested the Legislative Reference Bureau to analyze the potential impact on state government of decriminalizing certain offenses regarding the illegal possession of drugs. The Bureau requested information from federal, state, county, and private entities and individuals to complete this study. The Bureau extends its appreciation to all those that generously provided information and assistance in the preparation of this report. Charlotte A. Carter-Yamauchi Director January 2017 iii Table of Contents Page FOREWORD ......................................................................................................................... iii EXECUTIVE SUMMARY .................................................................................................. -

Harmonized Tariff Schedule of the United States (2020) Revision 7 Annotated for Statistical Reporting Purposes
Harmonized Tariff Schedule of the United States (2020) Revision 7 Annotated for Statistical Reporting Purposes CHAPTER 29 ORGANIC CHEMICALS VI 29-1 Notes 1. Except where the context otherwise requires, the headings of this chapter apply only to: (a) Separate chemically defined organic compounds, whether or not containing impurities; (b) Mixtures of two or more isomers of the same organic compound (whether or not containing impurities), except mixtures of acyclic hydrocarbon isomers (other than stereoisomers), whether or not saturated (chapter 27); (c) The products of headings 2936 to 2939 or the sugar ethers, sugar acetals and sugar esters, and their salts, of heading 2940, or the products of heading 2941, whether or not chemically defined; (d) Products mentioned in (a), (b) or (c) above dissolved in water; (e) Products mentioned in (a), (b) or (c) above dissolved in other solvents provided that the solution constitutes a normal and necessary method of putting up these products adopted solely for reasons of safety or for transport and that the solvent does not render the product particularly suitable for specific use rather than for general use; (f) The products mentioned in (a), (b), (c), (d) or (e) above with an added stabilizer (including an anticaking agent) necessary for their preservation or transport; (g) The products mentioned in (a), (b), (c), (d), (e) or (f) above with an added antidusting agent or a coloring or odoriferous substance added to facilitate their identification or for safety reasons, provided that the additions do not render the product particularly suitable for specific use rather than for general use; (h) The following products, diluted to standard strengths, for the production of azo dyes: diazonium salts, couplers used for these salts and diazotizable amines and their salts. -

HOUSE BILL 2368 by Mccormick SENATE BILL 2230 by Norris AN
HOUSE BILL 2368 By McCormick SENATE BILL 2230 By Norris AN ACT to amend Tennessee Code Annotated, Title 39, Chapter 17, Part 4, relative to the scheduling of controlled substances. BE IT ENACTED BY THE GENERAL ASSEMBLY OF THE STATE OF TENNESSEE: SECTION 1. Tennessee Code Annotated, Section 39-17-406, is amended by deleting the section in its entirety and substituting instead the following: 39-17-406 (a) Schedule I consists of the drugs and other substances, by whatever official name, common or usual name, chemical name, or brand name designated, listed in this section. (b) Opiates, unless specifically excepted or unless listed in another schedule, means any of the following opiates, including their isomers, esters, ethers, salts and salts of isomers, esters, and ethers, whenever the existence of such isomers, esters, ethers, and salts is possible within the specific chemical designation. For the purposes of subparagraph (b)(34) only, the term isomer includes the optical and geometric isomers. (1) Acetyl-alpha-methylfentanyl (N-[1-(1-methyl-2-phenethyl)-4- piperidinyl]-N-phenylacetamide); (2) Acetylmethadol; (3) Allylprodine; (4) Alphacetylmethadol (except levo-alphacetylmethadol also known as levo-alpha-acetylmethadol; levomethadyl acetate; or LAAM); (5) Alphameprodine; (6) Alphamethadol; SB2230 01065795 -1- (7) Alpha-methylfentanyl (N-[1-(alpha-methyl-beta-phenyl)ethyl-4- piperidyl]propionanilide; 1-(1-methyl-2-phenylethyl)-4-(N-propanilido)piperidine; (8) Alpha-methylthiofentanyl (N-[1-methyl-2-(2-thienyl) ethyl-4- piperidinyl]-N-phenylpropanamide); -
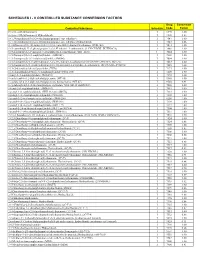
Controlled Substance Conversion Factors
SCHEDULES I - V CONTROLLED SUBSTANCE CONVERSION FACTORS Drug Conversion Controlled Substance Schedule Code Factor (+/-)cis -4-Methylaminorex I 1590 1.00 (+/-)cis -4-Methylaminorex Hydrochloride I 1590 0.83 1-(1,3-benzodioxol-5-yl)-2-(ethylamino)propan-1-one (ethylone) I 7547 1.00 1-(1,3-benzodioxol-5-yl)-2-(ethylamino)propan-1-one (ethylone) hydrochloride I 7547 0.86 (1-(4-fluorobenzyl)-1H -indol-3-yl)(2,2,3,3-tetramethylcyclopropyl)methanone (FUB-144) I 7014 1.00 1-(4-cyanobutyl)-N -(2-phenylpropan-2-yl)-1H -indazole-3-carboxamide (4-CN-CUMYL-BUTINACA) I 7089 1.00 1-(5-fluoropentyl)-1H -indazol-3-yl](naphthalen-1-yl)methanone (THJ–2201) 1 7024 1.00 1-(5-fluoropentyl)-3-(1-naphthoyl)indole (AM2201) I 7201 1.00 1-(5-fluoropentyl)-3-(2-iodobenzoyl)indole (AM694) I 7694 1.00 1-(5-fluoropentyl)-N -(2-phenylpropan-2-yl)-1H -indazole-3-carboxamide (5F-CUMYL-PINACA; SGT-25) I 7083 1.00 1-(5-fluoropentyl)-N -(2-phenylpropan-2-yl)-1H -pyrrolo[2,3-b]pyridine-3-carboxamide (5F-CUMYL-P7AICA) I 7085 1.00 1-[1-(2-thienyl)cyclohexyl]pyrrolidine (TCPy) I 7473 1.00 1-[2-(4-morpholinyl)ethyl]-3-(1-naphthoyl)indole (JWH- 200) I 7200 1.00 1-butyl-3-(1-naphthoyl)indole (JWH-073) I 7173 1.00 1-cyclohexyl-4-(1,2-diphenylethyl)piperazine (MT-45) I 9560 1.00 1-cyclohexyl-4-(1,2-diphenylethyl)piperazine hydrochloride (MT-45) I 9560 0.91 1-cyclohexylethyl-3-(2-methoxyphenylacetyl)indole 7008 (SR-18 and RCS-8) I 7008 1.00 1-hexyl-3-(1-naphthoyl)indole (JWH-019) I 7019 1.00 1-pentyl-3-(1-naphthoyl)indole (JWH-018 and AM678) I 7118 1.00 1-pentyl-3-(2-chlorophenylacetyl)indole