N-Glycosylation of TREK-1/Hk2p2.1 Two-Pore-Domain Potassium (K2P) Channels
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Potassium Channels in Epilepsy
Downloaded from http://perspectivesinmedicine.cshlp.org/ on September 28, 2021 - Published by Cold Spring Harbor Laboratory Press Potassium Channels in Epilepsy Ru¨diger Ko¨hling and Jakob Wolfart Oscar Langendorff Institute of Physiology, University of Rostock, Rostock 18057, Germany Correspondence: [email protected] This review attempts to give a concise and up-to-date overview on the role of potassium channels in epilepsies. Their role can be defined from a genetic perspective, focusing on variants and de novo mutations identified in genetic studies or animal models with targeted, specific mutations in genes coding for a member of the large potassium channel family. In these genetic studies, a demonstrated functional link to hyperexcitability often remains elusive. However, their role can also be defined from a functional perspective, based on dy- namic, aggravating, or adaptive transcriptional and posttranslational alterations. In these cases, it often remains elusive whether the alteration is causal or merely incidental. With 80 potassium channel types, of which 10% are known to be associated with epilepsies (in humans) or a seizure phenotype (in animals), if genetically mutated, a comprehensive review is a challenging endeavor. This goal may seem all the more ambitious once the data on posttranslational alterations, found both in human tissue from epilepsy patients and in chronic or acute animal models, are included. We therefore summarize the literature, and expand only on key findings, particularly regarding functional alterations found in patient brain tissue and chronic animal models. INTRODUCTION TO POTASSIUM evolutionary appearance of voltage-gated so- CHANNELS dium (Nav)andcalcium (Cav)channels, Kchan- nels are further diversified in relation to their otassium (K) channels are related to epilepsy newer function, namely, keeping neuronal exci- Psyndromes on many different levels, ranging tation within limits (Anderson and Greenberg from direct control of neuronal excitability and 2001; Hille 2001). -

A Computational Approach for Defining a Signature of Β-Cell Golgi Stress in Diabetes Mellitus
Page 1 of 781 Diabetes A Computational Approach for Defining a Signature of β-Cell Golgi Stress in Diabetes Mellitus Robert N. Bone1,6,7, Olufunmilola Oyebamiji2, Sayali Talware2, Sharmila Selvaraj2, Preethi Krishnan3,6, Farooq Syed1,6,7, Huanmei Wu2, Carmella Evans-Molina 1,3,4,5,6,7,8* Departments of 1Pediatrics, 3Medicine, 4Anatomy, Cell Biology & Physiology, 5Biochemistry & Molecular Biology, the 6Center for Diabetes & Metabolic Diseases, and the 7Herman B. Wells Center for Pediatric Research, Indiana University School of Medicine, Indianapolis, IN 46202; 2Department of BioHealth Informatics, Indiana University-Purdue University Indianapolis, Indianapolis, IN, 46202; 8Roudebush VA Medical Center, Indianapolis, IN 46202. *Corresponding Author(s): Carmella Evans-Molina, MD, PhD ([email protected]) Indiana University School of Medicine, 635 Barnhill Drive, MS 2031A, Indianapolis, IN 46202, Telephone: (317) 274-4145, Fax (317) 274-4107 Running Title: Golgi Stress Response in Diabetes Word Count: 4358 Number of Figures: 6 Keywords: Golgi apparatus stress, Islets, β cell, Type 1 diabetes, Type 2 diabetes 1 Diabetes Publish Ahead of Print, published online August 20, 2020 Diabetes Page 2 of 781 ABSTRACT The Golgi apparatus (GA) is an important site of insulin processing and granule maturation, but whether GA organelle dysfunction and GA stress are present in the diabetic β-cell has not been tested. We utilized an informatics-based approach to develop a transcriptional signature of β-cell GA stress using existing RNA sequencing and microarray datasets generated using human islets from donors with diabetes and islets where type 1(T1D) and type 2 diabetes (T2D) had been modeled ex vivo. To narrow our results to GA-specific genes, we applied a filter set of 1,030 genes accepted as GA associated. -

Variants in the KCNE1 Or KCNE3 Gene and Risk of Ménière’S Disease: a Meta-Analysis
Journal of Vestibular Research 25 (2015) 211–218 211 DOI 10.3233/VES-160569 IOS Press Variants in the KCNE1 or KCNE3 gene and risk of Ménière’s disease: A meta-analysis Yuan-Jun Li, Zhan-Guo Jin and Xian-Rong Xu∗ The Center of Clinical Aviation Medicine, General Hospital of Air Force, Beijing, China Received 1 August 2015 Accepted 8 December 2015 Abstract. BACKGROUND: Ménière’s disease (MD) is defined as an idiopathic disorder of the inner ear characterized by the triad of tinnitus, vertigo, and sensorineural hearing loss. Although many studies have evaluated the association between variants in the KCNE1 or KCNE3 gene and MD risk, debates still exist. OBJECTIVE: Our aim is to evaluate the association between KCNE gene variants, including KCNE1 rs1805127 and KCNE3 rs2270676, and the risk of MD by a systematic review. METHODS: We searched the literature in PubMed, SCOPUS and EMBASE through May 2015. We calculated pooled odds ra- tios (OR) and 95% confidence intervals (CIs) using a fixed-effects model or a random-effects model for the risk to MD associated with different KCNE gene variants. The heterogeneity assumption decided the effect model. RESULTS: A total of three relevant studies, with 302 MD cases and 515 controls, were included in this meta-analysis. The results indicated that neither the KCNE1 rs1805127 variant (for G vs. A: OR = 0.724, 95%CI 0.320, 1.638, P = 0.438), nor the KCNE3 rs2270676 variant (for T vs. C: OR = 0.714, 95%CI 0.327, 1.559, P = 0.398) was associated with MD risk. -

Circular RNA Hsa Circ 0005114‑Mir‑142‑3P/Mir‑590‑5P‑ Adenomatous
ONCOLOGY LETTERS 21: 58, 2021 Circular RNA hsa_circ_0005114‑miR‑142‑3p/miR‑590‑5p‑ adenomatous polyposis coli protein axis as a potential target for treatment of glioma BO WEI1*, LE WANG2* and JINGWEI ZHAO1 1Department of Neurosurgery, China‑Japan Union Hospital of Jilin University, Changchun, Jilin 130033; 2Department of Ophthalmology, The First Hospital of Jilin University, Jilin University, Changchun, Jilin 130021, P.R. China Received September 12, 2019; Accepted October 22, 2020 DOI: 10.3892/ol.2020.12320 Abstract. Glioma is the most common type of brain tumor APC expression with a good overall survival rate. UALCAN and is associated with a high mortality rate. Despite recent analysis using TCGA data of glioblastoma multiforme and the advances in treatment options, the overall prognosis in patients GSE25632 and GSE103229 microarray datasets showed that with glioma remains poor. Studies have suggested that circular hsa‑miR‑142‑3p/hsa‑miR‑590‑5p was upregulated and APC (circ)RNAs serve important roles in the development and was downregulated. Thus, hsa‑miR‑142‑3p/hsa‑miR‑590‑5p‑ progression of glioma and may have potential as therapeutic APC‑related circ/ceRNA axes may be important in glioma, targets. However, the expression profiles of circRNAs and their and hsa_circ_0005114 interacted with both of these miRNAs. functions in glioma have rarely been studied. The present study Functional analysis showed that hsa_circ_0005114 was aimed to screen differentially expressed circRNAs (DECs) involved in insulin secretion, while APC was associated with between glioma and normal brain tissues using sequencing the Wnt signaling pathway. In conclusion, hsa_circ_0005114‑ data collected from the Gene Expression Omnibus database miR‑142‑3p/miR‑590‑5p‑APC ceRNA axes may be potential (GSE86202 and GSE92322 datasets) and explain their mecha‑ targets for the treatment of glioma. -

Mutant Three-Repeat Tau Expression Initiates Retinal Ganglion Cell Death Through Caspase-2
UC San Diego UC San Diego Previously Published Works Title Mutant three-repeat tau expression initiates retinal ganglion cell death through Caspase-2. Permalink https://escholarship.org/uc/item/4gr7f6tn Authors Ngolab, Jennifer Canchi, Saranya Rasool, Suhail et al. Publication Date 2021-05-01 DOI 10.1016/j.nbd.2021.105277 Peer reviewed eScholarship.org Powered by the California Digital Library University of California HHS Public Access Author manuscript Author ManuscriptAuthor Manuscript Author Neurobiol Manuscript Author Dis. Author manuscript; Manuscript Author available in PMC 2021 August 18. Published in final edited form as: Neurobiol Dis. 2021 May ; 152: 105277. doi:10.1016/j.nbd.2021.105277. Mutant three-repeat tau expression initiates retinal ganglion cell death through Caspase-2 Jennifer Ngolaba, Saranya Canchia,c, Suhail Rasoola,d, Abderrahman Elmaaroufb, Kimberly Thomasb, Floyd Sarsozaa,c, Jennifer Grundmana, Michael Mantea, Jazmin Florioa, Nimisha Nandankara, Shaina Korouria, Wagner Zagob, Eliezer Masliahe, Robert A. Rissmana,c,* aDepartment of Neurosciences, University of California, San Diego, School of Medicine, La Jolla, CA 92093, United States of America bProthena Biosciences, South San Francisco, CA 94080, United States of America cVeterans Affairs San Diego Healthcare System, San Diego, CA 92161, United States of America dAmydis Inc, San Diego, CA 92121, United States of America eDivision of Neuroscience and Laboratory of Neurogenetics, National Institutes on Aging, NIH, Bethesda, MD 20892, United States of America Abstract The microtubule-associated protein tau is implicated in multiple degenerative diseases including retinal diseases such as glaucoma; however, the way tau initiates retinopathy is unclear. Previous retinal assessments in mouse models of tauopathy suggest that mutations in four-repeat (4R) tau are associated with disease-induced retinal dysfunction, while shifting tau isoform ratio to favor three-repeat (3R) tau production enhanced photoreceptor function. -

KCNE1 and KCNE3 Modulate KCNQ1 Channels by Affecting
KCNE1 and KCNE3 modulate KCNQ1 channels by PNAS PLUS affecting different gating transitions Rene Barro-Soriaa,1,2, Rosamary Ramentola, Sara I. Liina,3, Marta E. Pereza, Robert S. Kassb, and H. Peter Larssona,2 aDepartment of Physiology and Biophysics, Miller School of Medicine, University of Miami, Miami, FL 33136; and bDepartment of Pharmacology, College of Physicians and Surgeons, Columbia University, New York, NY 10032 Edited by Ramon Latorre, Universidad de Valparaíso, Valparaíso, Chile, and approved July 19, 2017 (received for review June 16, 2017) KCNE β-subunits assemble with and modulate the properties of on the activation of KCNQ1 channels is still debated. Here, we voltage-gated K+ channels. In the heart, KCNE1 associates with simultaneously track changes in voltage sensor movement and in the α-subunit KCNQ1 to generate the slowly activating, voltage- gate opening of KCNQ1/KCNE1 and KCNQ1/KCNE3 channels dependent potassium current (IKs) in the heart that controls the using voltage clamp fluorometry (VCF) to determine the differ- repolarization phase of cardiac action potentials. By contrast, in ences in the molecular mechanisms by which KCNE1 and KCNE3 epithelial cells from the colon, stomach, and kidney, KCNE3 coas- alter KCNQ1 channel gating. + sembles with KCNQ1 to form K channels that are voltage- Pore-forming KCNQ1 channel α-subunits form tetramers. Each + independent K channels in the physiological voltage range and α-subunit has six transmembrane (TM) segments (S1–S6) divided important for controlling water and salt secretion and absorption. into two functional domains: a voltage sensor domain comprising How KCNE1 and KCNE3 subunits modify KCNQ1 channel gating so four peripheral TM helices (S1–S4) and a centrally located pore differently is largely unknown. -
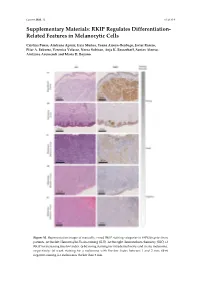
RKIP Regulates Differentiation- Related Features in Melanocytic Cells
Cancers 2020. 12 S1 of S14 Supplementary Materials: RKIP Regulates Differentiation- Related Features in Melanocytic Cells Cristina Penas, Aintzane Apraiz, Iraia Muñoa, Yoana Arroyo-Berdugo, Javier Rasero, Pilar A. Ezkurra, Veronica Velasco, Nerea Subiran, Anja K. Bosserhoff, Santos Alonso, Aintzane Asumendi and Maria D. Boyano Figure S1. Representative images of manually scored RKIP staining categories in FFPE biopsies from patients. At the feft: Hematoxylin-Eosin staining (H-E). At the right: Immunohistochemistry (IHC) of RKIP for increasing Breslow Index. (a-b) Strong staining for intradermal nevus and in situ melanoma, respectively; (c) weak staining for a melanoma with Breslow Index between 1 and 2 mm; (d-e) negative staining for melanomas thicker than 2 mm. Cancers 2020. 12 S2 of S14 Figure S2. RNA Sequencing data analysis. Each row of the figure represents an over-represented gene signature generated by analyzing the up-regulated and down-regulated genes from a differential expression analysis of RKIP Knockdown melanocytes HEMn-LP. Results from the EnrichR Website with a p-value > 0.05. Figure S3. RNA Sequencing data quality. (a) Read counts normalization per sample; (b) Spearman correlation coefficient between replicates; (c) Principal component analysis for replicates. Cancers 2020. 12 S3 of S14 Figure S4. Details of Western Blot from Figure 2 related with RKIP expression in several cell lines. (a) Blots for RKIP and Tubulin in primary and metastatic melanoma and in three primary melanocytes HEMn-DP (dark pigmented), HEMn-LP (light pigmented) and HEM2710. On the right, original blot for each analyzed protein. Highlighted with a red square the information included in the Figure 3 (horizontal rotation). -

Ion Channels 3 1
r r r Cell Signalling Biology Michael J. Berridge Module 3 Ion Channels 3 1 Module 3 Ion Channels Synopsis Ion channels have two main signalling functions: either they can generate second messengers or they can function as effectors by responding to such messengers. Their role in signal generation is mainly centred on the Ca2 + signalling pathway, which has a large number of Ca2+ entry channels and internal Ca2+ release channels, both of which contribute to the generation of Ca2 + signals. Ion channels are also important effectors in that they mediate the action of different intracellular signalling pathways. There are a large number of K+ channels and many of these function in different + aspects of cell signalling. The voltage-dependent K (KV) channels regulate membrane potential and + excitability. The inward rectifier K (Kir) channel family has a number of important groups of channels + + such as the G protein-gated inward rectifier K (GIRK) channels and the ATP-sensitive K (KATP) + + channels. The two-pore domain K (K2P) channels are responsible for the large background K current. Some of the actions of Ca2 + are carried out by Ca2+-sensitive K+ channels and Ca2+-sensitive Cl − channels. The latter are members of a large group of chloride channels and transporters with multiple functions. There is a large family of ATP-binding cassette (ABC) transporters some of which have a signalling role in that they extrude signalling components from the cell. One of the ABC transporters is the cystic − − fibrosis transmembrane conductance regulator (CFTR) that conducts anions (Cl and HCO3 )and contributes to the osmotic gradient for the parallel flow of water in various transporting epithelia. -

Ion Channels As Part of Macromolecular Multiprotein Complexes Clinical Significance
Schwerpunkt Herzschr Elektrophys 2018 · 29:30–35 Jordi Heijman1 · Dobromir Dobrev2 https://doi.org/10.1007/s00399-017-0542-y 1 Department of Cardiology, Cardiovascular Research Institute Maastricht, Faculty of Health, Medicine, and Received: 9 August 2017 Life Sciences, Maastricht University, Maastricht, The Netherlands Accepted: 11 October 2017 2 Institute of Pharmacology, West German Heart and Vascular Center, Faculty of Medicine, University Published online: 6 December 2017 Duisburg-Essen, Essen, Germany © The Author(s) 2017. This article is an open access publication. Ion channels as part of macromolecular multiprotein complexes Clinical significance Introduction Cardiac cellular electrophysiol- Na+ and K+ concentrations is achieved ogy and arrhythmogenesis via the Na+-K+-ATPase. Every heartbeat is orchestrated by a cas- Each of these ion channels is in fact cade of electrical activity that initiates Although there are important quantita- a large macromolecular complex consist- contraction in cardiomyocytes through tive differences in cellular electrophys- ingofnumerousproteinsthatregulate a process termed excitation–contraction iology and Ca2+ handling between dif- the intracellular movement and distri- coupling [1, 2]. The electrophysiological ferent cardiac regions (reviewed in [1, bution (a processes termed trafficking) properties of cardiomyocytes are dy- 2, 4]), a number of commonalities and and function of these channels. Dys- namically regulated to adapt to varying general mechanisms can be highlighted. function of any of these ion channels demands. Research performed during The upstroke of the action potential (AP) in the setting of cardiovascular disease the past 20 years has shown that the ion in cardiomyocytes is mediated by Na+ may predispose to atrial or ventricular channelsand Ca2+-handlingproteinsthat influx through voltage-dependent Na+ arrhythmias by promoting triggered ac- are essential for cardiomyocyte electro- channels. -

Physiological and Pathophysiological Regulation of the Ryanodine Receptor in Skeletal Muscle
Physiological and pathophysiological regulation of the ryanodine receptor in skeletal muscle Alisa Umanskaya Submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy in the Graduate School of Arts and Sciences COLUMBIA UNIVERSITY 2015 © 2015 Alisa Umanskaya All rights reserved Abstract Physiological and pathophysiological regulation of ryanodine receptor in skeletal muscle Alisa Umanskaya Ryanodine receptor calcium release channels are essential for skeletal muscle contraction, as they mediate the release of calcium ions from intracellular stores into the cytosol. The data presented in this dissertation demonstrate the evolutionarily conserved mechanisms of skeletal muscle ryanodine receptor regulation in the physiological and pathophysiological states. Adrenergic stimulation causes increased skeletal muscle force, however, despite the well- established role of this physiological response, the molecular mechanism is not known. Here we present a mechanism whereby phosphorylation of a single amino acid on the ryanodine receptor is a key signal in the physiological stress-induced inotropic response in mouse skeletal muscle. Therefore acute post-translational modifications of ryanodine receptor channels are important for healthy muscle contraction. Conversely, chronic stress-induced post-translational modifications result in poorly functioning murine ryanodine receptor channels that contribute to skeletal muscle dysfunction in age- dependent skeletal muscle weakness and Muscular Dystrophies. Finally, we present data that demonstrates striking evolutionary conservation in ryanodine receptor regulation in the physiological and pathophysiological states between mice and C. elegans. This work has broad implications for understanding the underlying mechanisms of skeletal muscle contraction and important disorders that affect human health. Furthermore, this works presents ryanodine receptor channels as a viable therapeutic target for age-related skeletal muscle weakness, Muscular Dystrophies, and also implicates C. -

Stem Cells and Ion Channels
Stem Cells International Stem Cells and Ion Channels Guest Editors: Stefan Liebau, Alexander Kleger, Michael Levin, and Shan Ping Yu Stem Cells and Ion Channels Stem Cells International Stem Cells and Ion Channels Guest Editors: Stefan Liebau, Alexander Kleger, Michael Levin, and Shan Ping Yu Copyright © 2013 Hindawi Publishing Corporation. All rights reserved. This is a special issue published in “Stem Cells International.” All articles are open access articles distributed under the Creative Com- mons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Editorial Board Nadire N. Ali, UK Joseph Itskovitz-Eldor, Israel Pranela Rameshwar, USA Anthony Atala, USA Pavla Jendelova, Czech Republic Hannele T. Ruohola-Baker, USA Nissim Benvenisty, Israel Arne Jensen, Germany D. S. Sakaguchi, USA Kenneth Boheler, USA Sue Kimber, UK Paul R. Sanberg, USA Dominique Bonnet, UK Mark D. Kirk, USA Paul T. Sharpe, UK B. Bunnell, USA Gary E. Lyons, USA Ashok Shetty, USA Kevin D. Bunting, USA Athanasios Mantalaris, UK Igor Slukvin, USA Richard K. Burt, USA Pilar Martin-Duque, Spain Ann Steele, USA Gerald A. Colvin, USA EvaMezey,USA Alexander Storch, Germany Stephen Dalton, USA Karim Nayernia, UK Marc Turner, UK Leonard M. Eisenberg, USA K. Sue O’Shea, USA Su-Chun Zhang, USA Marina Emborg, USA J. Parent, USA Weian Zhao, USA Josef Fulka, Czech Republic Bruno Peault, USA Joel C. Glover, Norway Stefan Przyborski, UK Contents Stem Cells and Ion Channels, Stefan Liebau, -

Mechanisms Underlying Kcnq1channel Cell Volume Sensitivity
THE PHD SCHOOL OF SC IENCE FACULTY OF SCIENCE UNIVERSITY OF COPENHAGEN PhD thesis Sofia Hammami Mechanisms underlying KCNQ1channel cell volume sensitivity Submitted: 10/05/10 1 TABLE OF CONTENTS Preface ............................................................................................................................................................... 5 Acknowledgements.......................................................................................................................................... 5 Publications ....................................................................................................................................................... 6 Summary ............................................................................................................................................................ 7 Dansk resumé ................................................................................................................................................... 8 Abbreviations ................................................................................................................................................... 9 Table of figures .............................................................................................................................................. 10 BACKGROUND .............................................................................................................................. 11 Ion Channels ..................................................................................................................................................