2014 Skeleton Lake Report
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Summary Report of the Geological Survey for the Calendar Year 1911
5 GEORGE V. SESSIONAL PAPER No. 26 A. 1915 SUMMARY REPORT OK THE GEOLOGICAL SURVEY DEPARTMENT OF MINES FOR THE CALENDAR YEAR 1914 PRINTED BY ORDER OF PARLIAMENT. OTTAWA PRTNTKD BY J. i»k L TAOHE, PRINTER TO THE KING'S MOST EXCELLENT IfAJESTS [No. 26—1915] [No , 15031 5 GEORGE V. SESSIONAL PAPER No. 26 A. 1915 To Field Marshal, Hit Hoi/al Highness Prince Arthur William Patrick Albert, Duke of Connaught and of Strath-earn, K.G., K.T., K.P., etc., etc., etc., Governor General and Commander in Chief of the Dominion of Canada. May it Please Youb Royal Highness.,— The undersigned has the honour to lay before Your Royal Highness— in com- pliance with t>-7 Edward YIT, chapter 29, section IS— the Summary Report of the operations of the Geological Survey during the calendar year 1914. LOUIS CODERRK, Minister of Mines. 5 GEORGE V. SESSIONAL PAPER No. 26 A. 1915 To the Hon. Louis Codebrk, M.P., Minister of Mines, Ottawa. Sir,—I have the honour to transmit, herewith, my summary report of the opera- tions of the Geological Survey for the calendar year 1914, which includes the report* of the various officials on the work accomplished by them. I have the honour to be, sir, Your obedient servant, R. G. MrCOXXFI.L, Deputy Minister, Department of Mines. B . SESSIONAL PAPER No. 28 A. 1915 5 GEORGE V. CONTENTS. Paok. 1 DIRECTORS REPORT REPORTS FROM GEOLOGICAL DIVISION Cairncs Yukon : D. D. Exploration in southwestern "" ^ D. MacKenzie '\ Graham island. B.C.: J. M 37 B.C. -

The Archaeology of Brabant Lake
THE ARCHAEOLOGY OF BRABANT LAKE A Thesis Submitted to the College of Graduate Studies and Research in Partial Fulfilment of the Requirements for the Degree of Master of Arts in the Department of Anthropology and Archaeology University of Saskatchewan Saskatoon By Sandra Pearl Pentney Fall 2002 © Copyright Sandra Pearl Pentney All rights reserved. PERMISSION TO USE PERMISSION TO USE In presenting this thesis in partial fulfilment of the requirements for a Postgraduate degree from the University of Saskatchewan, I agree that the Libraries of this University may make it freely available for inspection. I further agree that permission for copying of this thesis in any manner, in whole or in part, for scholarly purposes may be granted by the professor or professors who supervised my thesis work or, In their absence, by the Head of the Department or the Dean of the College in which my thesis work was done. It is understood that any copying or publication or use of this thesis or parts thereof for financial gain shall not be allowed without my written permission. It is also understood that due recognition shall be given to me and to the University of Saskatchewan in any scholarly use which may be made of any material in my thesis. Requests for permission to copy or to make other use of material in this thesis in whole or part should be addressed to: Head of the Department of Anthropology and Archaeology University of Saskatchewan Saskatoon, Saskatchewan (S7N 5B 1) ABSTRACT Boreal forest archaeology is costly and difficult because of rugged terrain, the remote nature of much of the boreal areas, and the large expanses of muskeg. -

1E'r County Counci 1 Meeting
I ;;>fee.:<"" t�, . R�§l'1e'r County Counci 1 Meeting . c·ounty of Athabas. ca �o. 12 Tuesday, July 24, 1984 Present: Reeve Kostil•<; Councillors Flasha, Bencharsky, Le>�is, Keith (A./1. only), tlatoga, Johnson, Patry and Richards; County Administrator L. l. Gurel; Assistant Secretary-Treasurer H. R. Jodry; Hunictpal Intern 8. Waldowski. Ca II to Order: Reeve r<osti•' called the meeting to order at 10:00 A.M. �li nutes: It was moved by Councillor Richards that the minutes of the regular County Counci1 meeting of Ju11e 28, 1984, be ap�r.oveld as corrected. Hotion carried. Corr.ection on Page 6498 under heading Drainage - Amlsk Lake. Amend motion as follows: "It by the •1as moved Counci IIor Flasha to amend .agreement for Charron Creek from $40,000. to ·$65;000. and .... " It was moved by Counct llor Flasha that the minutes of the regular County Council meeting of July 3, 1984, be approved as corrected. Motion carried. Correction on Page 65DI under heading Family & ·community Support Services. Amend sentence as· fo 110\•IS: .. "Counci Ilor ·Patry reported Aganda: It •1as moved by Counci Ilor Hatoga that the agenda, wl th additions, be accepted by Counci I. Motion carried unanimously. 1•\tnutes : It •<as moved by Counci Ilor Bencharsky that the minutes of the special County Council meeting of July 6, 1984, be approved as corrected. /•lotion carried. Correction under heading \�estern ·rractot· as follo1·1s: "Harranty on elther Grader - 6 months ••." De Iega t ion: R. Keglowitsch, County A.L.S., attended the meeting at this time to . -

RURAL ECONOMY Ciecnmiiuationofsiishiaig Activity Uthern All
RURAL ECONOMY ciEcnmiIuationofsIishiaig Activity uthern All W Adamowicz, P. BoxaIl, D. Watson and T PLtcrs I I Project Report 92-01 PROJECT REPORT Departmnt of Rural [conom F It R \ ,r u1tur o A Socio-Economic Evaluation of Sportsfishing Activity in Southern Alberta W. Adamowicz, P. Boxall, D. Watson and T. Peters Project Report 92-01 The authors are Associate Professor, Department of Rural Economy, University of Alberta, Edmonton; Forest Economist, Forestry Canada, Edmonton; Research Associate, Department of Rural Economy, University of Alberta, Edmonton and Research Associate, Department of Rural Economy, University of Alberta, Edmonton. A Socio-Economic Evaluation of Sportsfishing Activity in Southern Alberta Interim Project Report INTROI)UCTION Recreational fishing is one of the most important recreational activities in Alberta. The report on Sports Fishing in Alberta, 1985, states that over 340,000 angling licences were purchased in the province and the total population of anglers exceeded 430,000. Approximately 5.4 million angler days were spent in Alberta and over $130 million was spent on fishing related activities. Clearly, sportsfishing is an important recreational activity and the fishery resource is the source of significant social benefits. A National Angler Survey is conducted every five years. However, the results of this survey are broad and aggregate in nature insofar that they do not address issues about specific sites. It is the purpose of this study to examine in detail the characteristics of anglers, and angling site choices, in the Southern region of Alberta. Fish and Wildlife agencies have collected considerable amounts of bio-physical information on fish habitat, water quality, biology and ecology. -

Jackfish Lake State of the Watershed Report
Jackfish Lake State of the Watershed Report April 2016 North Saskatchewan Watershed Alliance 202 - 9440 49th St NW Edmonton, AB T6B 2M9 Tel: (587) 525-6820 Email: [email protected] http://www.nswa.ab.ca The North Saskatchewan Watershed Alliance (NSWA) is a non-profit society whose purpose is to protect and improve water quality and ecosystem functioning in the North Saskatchewan River watershed in Alberta. The organization is guided by a Board of Directors composed of member organizations from within the watershed. It is the designated Watershed Planning and Advisory Council (WPAC) for the North Saskatchewan River under the Government of Alberta’s Water for Life Strategy.. This report was prepared by Jennifer Regier, B.Sc. and David Trew, P.Biol of the North Saskatchewan Watershed Alliance. Cover photo credit: Dara Choy, Stony Plain AB Suggested Citation: North Saskatchewan Watershed Alliance (NSWA). 2016. Jackfish Lake State of the Watershed Report. Prepared by the NSWA, Edmonton, AB. for the Jackfish Lake Management Association, Carvel, AB. Available on the internet at http://www.nswa.ab.ca/resources/ nswa_publications Jackfish Lake State of the Watershed Report Executive Summary The purpose of this report is to consolidate environmental information on Jackfish Lake and its watershed in an effort to support future planning and management discussions. The report provides perspective on current environmental conditions at the lake relative to regional and historic trends. The report is provided as advice to the Jackfish Lake Management Association (JLMA), Alberta Environment and Parks, and Parkland County. The technical information contained in this document is detailed and addresses many lake and watershed features. -

Mayatan Lake State of the Watershed Report
Mayatan Lake State of the Watershed Report August 2012 Mayatan Lake State of the Watershed Report North Saskatchewan Watershed Alliance 9504‐49 Street Edmonton, AB T6B 2M9 Tel: (780) 442‐6363 Fax: (780) 495‐0610 Email: [email protected] http://www.nswa.ab.ca The North Saskatchewan Watershed Alliance (NSWA) is a non‐profit society whose purpose is to protect and improve water quality and ecosystem functioning in the North Saskatchewan River watershed in Alberta. The organization is guided by a Board of Directors composed of member organizations from within the watershed. It is the designated Watershed Planning and Advisory Council (WPAC) for the North Saskatchewan River under the Government of Alberta’s Water for Life Strategy. This report was prepared by Melissa Logan, P.Biol., Billie Milholland, B.A., and David Trew, P.Biol., of the North Saskatchewan Watershed Alliance. Suggested Citation: North Saskatchewan Watershed Alliance (NSWA). 2012. Mayatan Lake State of the Watershed Report. Prepared by the NSWA, Edmonton, AB., for the Mayatan Lake Management Association, Carvel, AB. Available on the internet at http://www.nswa.ab.ca/resources/nswa_publications 1 Mayatan Lake State of the Watershed Report Acknowledgements The NSWA gratefully acknowledges the contribution of the following persons towards the completion of this report: Yaw Okyere and Janet Yan of Alberta Environment for conducting a search of water allocations within the basin; Terry Chamuluk of Alberta Environment for providing Morton monthly evaporation estimates for Alberta; Ron Woodvine of AAFC for providing precipitation and evaporation tables and maps for the Canadian Prairies; Rick Rickwood and Candace Vanin of AAFC for providing maps and delineating the gross and effective areas of Mayatan Lake; Graham Watt for preparing maps; and Sal Figliuzzi for completion of the water balance for the lake. -

Lake Stewardship Reference Guide
Association of Summer Villages of Alberta Association of Summer Villages of Alberta Lake Stewardship ReferenceReference GuideGuide Association of Summer Villages of Alberta 2006 Edition Acknowledgements The Association of Summer Villages of Alberta (ASVA), representing Alberta’s Summer Village municipalities, was formed in 1977. The ASVA advocates on common Summer Village issues, proposes solutions, and provides education for Summer Village Councils. The ASVA has long maintained that every Summer Village is dealing with lake stewardship issues today, and will continue to deal with them in the future. Through this Lake Stewardship Reference Guide, the ASVA has taken a leadership role in the stewardship of our lakes in partnership with other provincial government departments, organizations, and agencies. The Lake Stewardship Reference Guide was prepared through the ASVA Lake Stewardship Steering Committee. Members of this Lake Stewardship Steering Committee include: Bruce McIntosh, Chair, Summer Village of Island Lake Susan Evans-Dzus, Summer Villages of Seba Beach and Lakeview Art Lamoureux, Summer Village of White Sands Bob Lindsay, Summer Village of Sunset Beach Lori Jeffery-Heaney, Summer Village of Val Quentin, Alberta Urban Municipalities Association Director of Summer Villages Jim Sandmaier, Summer Village of Island Lake South Peter Wright, Summer Village of Sunrise Beach Special thanks to the following individuals who participated in preparing this Reference Guide: Gerry Haekel, Alberta Sustainable Resource Development Shelleen Lakusta, Alberta Environment Christine Lazaruk, Alberta Urban Municipalities Association, Alberta Environment Terry Sly, Alberta Environment Bruce Thom, Alberta Urban Municipalities Association Island Lake (front cover) photo credit: Jim Sandmaier Contributing authors and editors: Pat Valastin and Lori Jeffery-Heaney Design and graphic layout services: Pat Valastin and Hound’s Tooth Communications Ltd. -
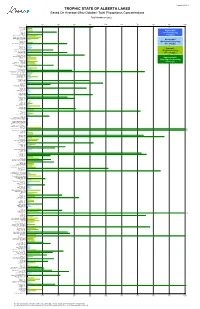
Trophic State of Alberta Lakes Based on Average Total Phosphorus
Created Feb 2013 TROPHIC STATE OF ALBERTA LAKES Based On Average (May-October) Total Phosphorus Concentrations Total Phosphorus (µg/L) 0 100 200 300 400 500 600 700 800 900 1000 * Adamson Lake Alix Lake * Amisk Lake * Angling Lake Oligotrophic * ‡ Antler Lake Arm Lake (Low Productivity) * Astotin Lake (<10 µg/L) * ‡ Athabasca (Lake) - Off Delta Baptiste Lake - North Basin Baptiste Lake - South Basin * ‡ Bare Creek Res. Mesotrophic * ‡ Barrier Lake ‡ Battle Lake (Moderate Productivity) * † Battle River Res. (Forestburg) (10 - 35 µg/L) Beartrap Lake Beauvais Lake Beaver Lake * Bellevue Lake Eutrophic * † Big Lake - East Basin * † Big Lake - West Basin (High Productivity) * Blackfalds Lake (35 - 100 µg/L) * † Blackmud Lake * ‡ Blood Indian Res. Bluet (South Garnier Lake) ‡ Bonnie Lake Hypereutrophic † Borden Lake * ‡ Bourque Lake (Very High Productivity) ‡ Buck Lake (>100 µg/L) Buffalo Lake - Main Basin Buffalo Lake - Secondary Bay * † Buffalo Lake (By Boyle) † Burntstick Lake Calling Lake * † Capt Eyre Lake † Cardinal Lake * ‡ Carolside Res. - Berry Creek Res. † Chain Lakes Res. - North Basin † Chain Lakes Res.- South Basin Chestermere Lake * † Chickakoo Lake * † Chickenhill Lake * Chin Coulee Res. * Clairmont Lake Clear (Barns) Lake Clear Lake ‡ Coal Lake * ‡ Cold Lake - English Bay ‡ Cold Lake - West Side ‡ Cooking Lake † Cow Lake * Crawling Valley Res. Crimson Lake Crowsnest Lake * † Cutbank Lake Dillberry Lake * Driedmeat Lake ‡ Eagle Lake ‡ Elbow Lake Elkwater Lake Ethel Lake * Fawcett Lake * † Fickle Lake * † Figure Eight Lake * Fishing Lake * Flyingshot Lake * Fork Lake * ‡ Fox Lake Res. Frog Lake † Garner Lake Garnier Lake (North) * George Lake * † Ghost Res. - Inside Bay * † Ghost Res. - Inside Breakwater ‡ Ghost Res. - Near Cochrane * Gleniffer Lake (Dickson Res.) * † Glenmore Res. -

Western Grebe Surveys in Alberta 2016
WESTERN GREBE SURVEYS IN ALBERTA 2016 The western grebe has been listed as a Threatened species in Alberta. A recent data compilation shows that there are approximately 250 lakes that have supported western grebes in Alberta. However, information for most lakes is poor and outdate d. Total counts on lakes are rare, breeding status is uncertain, and the location and extent of breeding habitat (emergent vegetation, usually bulrush) is usually unknown. We are seeking your help in gathering more information on western grebe populations in Alberta. If you visit any of the lakes listed below, or know anyone that does, we would appreciate as much detail as you can collect on the presence of western grebes and their habitat. Let us know in advance (if possible) if you are planning on going to any lakes, and when you do, e-mail details of your observations to [email protected]. SURVEY METHODS: Visit a lake between 1 May and 31 August with spotting scope or good binoculars. Surveys can be done from a boat, or vantage point(s) from shore. Report names of surveyors, dates, number of adults seen, and report on the approximate percentage of the lake area that this number represents. Record presence of young birds or nesting colonies, and provide any additional information on presence/location of likely breeding habitat, specific parts of the lake observed, observed threats to birds or habitat (boat traffic, shoreline clearing, pollution, etc.). Please report on findings even if no birds were seen. Lakes on the following page that are flagged with an asterisk (*) were not visited in 2015, and are priority for survey in 2016. -

Monitoring for Invasive Mussels in Alberta’S Irrigation Infrastructure: 2017 Report
Monitoring for Invasive Mussels in Alberta’s Irrigation Infrastructure: 2017 Report Alberta Agriculture and Forestry Water Quality Section Outlet of Sauder Reservoir January 2018 Introduction and Summary The Government of Alberta (GOA) is committed to protecting the province against aquatic invasive species (AIS), due to their negative ecological and economic effects. Invasive zebra mussels (Dreissena polymorpha) and quagga mussels (Dreissena bugensis) are of prominent concern, as these dreissenid mussels attach to any solid submerged surface and rapidly multiply due to their high reproductive rates. They are also very difficult to contain and eradicate once established. Additionally, they are spreading closer to Alberta’s borders. Alberta’s irrigation industry contributes $3.6 billion to the provincial gross domestic product (GDP). Specifically, it contributes about 20% of the provincial agri-food sector GDP on 4.7% of the province’s cultivated land base (Paterson Earth & Water Consulting 2015). Alberta’s irrigation industry includes thirteen irrigation districts that supply water to more than 570,000 ha of farmland through infrastructure valued at $3.6 billion. This infrastructure includes 57 irrigation reservoirs along with 3,491 km of canals and 4,102 km of pipelines (ARD 2014; AF 2017). The irrigation conveyance system provides water to irrigators, municipalities, industries, and wetlands, while the reservoirs support recreational activities such as boating and fishing and provide habitat to fish and waterfowl. Invasive mussels are a concern to the irrigation industry as infestations will have a significant negative effect on water infrastructure and conveyance works due to their ability to completely clog pipelines and damage raw-water treatment systems and intakes. -

Athabasca County Municipal Development Plan
Athabasca County Municipal Development Plan September 13, 2016 Final Draft Bylaw No. 013-2016 Submitted by: Submitted to: 2 of 110 ACKNOWLEDGEMENTS Municipal Planning Commission: Planning & Development: Jesse Ajayi, Director of Planning & Development Elaine Leeuw, Subdivision and Development Officer Consulting Team: Armin Preiksaitis, President Wayne Jackson, Senior Associate Karolina Drabik, Senior Planner 3 of 110 4 of 110 TABLE OF CONTENTS PART I | CONTEXT 9 1.0 Introduction 11 1.1 Nature of a Municipal Development Plan 12 1.2 Enabling Legislation 12 1.3 How the Municipal Development Plan was Developed (Planning Process) 14 1.4 Scope and Direction of Plan 15 1.5 Plan Organization 16 1.6 Plan Terminology 17 1.7 Plan Amendment 18 2.0 Regional Context and Emerging Trends 19 2.1 Regional Context 19 2.2 Historical Development 20 2.3 Population Growth and Change 20 2.4 Economic Development and Employment Growth 22 2.5 Population Growth Forecast 22 3.0 Vision Statement, Strategic Direction and Growth Management Strategy 25 3.1 Vision Statement 25 3.2 Strategic Direction 25 3.3 Growth Management Strategy 26 PART II | LAND USE PLANNING AND DEVELOPMENT 27 4.0 Agriculture and Rural Development 29 4.1 Background 29 4.2 Objectives 31 4.3 County Planning Policies 31 5.0 Residential Development 37 5.1 Background 37 5.2 Objectives 38 5.3 Rural Residential Subdivision and Development 38 5.4 Country Residential (Multi-lot) Subdivision and Development 40 5.5 Work Camps 43 6.0 Hamlet Development 45 6.1 Background 45 6.2 Objectives 46 6.3 County Planning -

The Second Report
The Second Report Watershed Stewardship Grant Program (Spring 2006, Fall 2006, Spring 2007) AENV Grant: #06GREA29 Sarah Hipkin & Kevin Wirtanen Grant Administrators 2006-2008 Alberta Stewardship Network Special thanks to: Alberta Environment Members of the Stewardship Grant Committee: Margaret Glasford, ASN Chair / Past Chair and Grant Committee Chair (all grant cycles) Petra Rowell, Alberta Environment (all) Jeff McCammon, Lac La Nonne Watershed and Lake Stewardship Society (all) Shirley Pickering, Highwood Water Management Plan Public Advisory Committee (all) Ernie Ewaschuk and Sarah Primeau, Land Stewardship Centre of Canada & ASN Secretariat Ken Lewis and Dale Chrapko, Alberta Agriculture and Food /AESA Diana Rung, Alberta Conservation Association (all) Kelsey Spicer-Rawe, Alberta Riparian Habitat Management Society (Cows & Fish) (all) Wendy Devent, Stephanie Palechek and Leda Kozak, Oldman Watershed Council Kent Lyle, Sylvan Lake Watershed Stewardship Society (Spring 2006) Frank Vagi, North Saskatchewan Watershed Alliance (Fall 2006) Jacqueline Nelson, ASN Chair (2007/8 on) and Foothills Land Trust (Spring 2008) Jodi Miller, Volunteer Steward (Spring 2008) Additional appreciation is extended to the ASN’s other partnering organizations. The delivery of the range of ASN services is dependent on their ongoing support. 2 CONTENTS The Ripple Reaches Further: The Second Report on the Watershed Stewardship Grant Program ..............................................................................6 Highlights Reach .............................................................................................................................................