Contractile Pericytes Determine the Direction of Blood Flow at Capillary Junctions
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Chapter 20 *Lecture Powerpoint the Circulatory System: Blood Vessels and Circulation
Chapter 20 *Lecture PowerPoint The Circulatory System: Blood Vessels and Circulation *See separate FlexArt PowerPoint slides for all figures and tables preinserted into PowerPoint without notes. Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Introduction • The route taken by the blood after it leaves the heart was a point of much confusion for many centuries – Chinese emperor Huang Ti (2697–2597 BC) believed that blood flowed in a complete circuit around the body and back to the heart – Roman physician Galen (129–c. 199) thought blood flowed back and forth like air; the liver created blood out of nutrients and organs consumed it – English physician William Harvey (1578–1657) did experimentation on circulation in snakes; birth of experimental physiology – After microscope was invented, blood and capillaries were discovered by van Leeuwenhoek and Malpighi 20-2 General Anatomy of the Blood Vessels • Expected Learning Outcomes – Describe the structure of a blood vessel. – Describe the different types of arteries, capillaries, and veins. – Trace the general route usually taken by the blood from the heart and back again. – Describe some variations on this route. 20-3 General Anatomy of the Blood Vessels Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Capillaries Artery: Tunica interna Tunica media Tunica externa Nerve Vein Figure 20.1a (a) 1 mm © The McGraw-Hill Companies, Inc./Dennis Strete, photographer • Arteries carry blood away from heart • Veins -
Cardiovascular System 9
Chapter Cardiovascular System 9 Learning Outcomes On completion of this chapter, you will be able to: 1. State the description and primary functions of the organs/structures of the car- diovascular system. 2. Explain the circulation of blood through the chambers of the heart. 3. Identify and locate the commonly used sites for taking a pulse. 4. Explain blood pressure. 5. Recognize terminology included in the ICD-10-CM. 6. Analyze, build, spell, and pronounce medical words. 7. Comprehend the drugs highlighted in this chapter. 8. Describe diagnostic and laboratory tests related to the cardiovascular system. 9. Identify and define selected abbreviations. 10. Apply your acquired knowledge of medical terms by successfully completing the Practical Application exercise. 255 Anatomy and Physiology The cardiovascular (CV) system, also called the circulatory system, circulates blood to all parts of the body by the action of the heart. This process provides the body’s cells with oxygen and nutritive ele- ments and removes waste materials and carbon dioxide. The heart, a muscular pump, is the central organ of the system. It beats approximately 100,000 times each day, pumping roughly 8,000 liters of blood, enough to fill about 8,500 quart-sized milk cartons. Arteries, veins, and capillaries comprise the network of vessels that transport blood (fluid consisting of blood cells and plasma) throughout the body. Blood flows through the heart, to the lungs, back to the heart, and on to the various body parts. Table 9.1 provides an at-a-glance look at the cardiovascular system. Figure 9.1 shows a schematic overview of the cardiovascular system. -

Molecular Signatures of Tissue-Specific
Developmental Cell Resource Molecular Signatures of Tissue-Specific Microvascular Endothelial Cell Heterogeneity in Organ Maintenance and Regeneration Daniel J. Nolan,1,6 Michael Ginsberg,1,6 Edo Israely,1 Brisa Palikuqi,1 Michael G. Poulos,1 Daylon James,1 Bi-Sen Ding,1 William Schachterle,1 Ying Liu,1 Zev Rosenwaks,2 Jason M. Butler,1 Jenny Xiang,4 Arash Rafii,1,7 Koji Shido,1 Sina Y. Rabbany,1,8 Olivier Elemento,3 and Shahin Rafii1,5,* 1Department of Genetic Medicine, Howard Hughes Medical Institute 2Ronald O. Perelman and Claudia Cohen Center for Reproductive Medicine 3HRH Prince Alwaleed Bin Talal Bin Abdulaziz Alsaud Institute for Computational Biomedicine 4Genomics Resource Core Facility Weill Cornell Medical College, New York, NY 10065, USA 5Ansary Stem Cell Institute, New York, NY 10065, USA 6Angiocrine Bioscience, New York, NY 10065, USA 7Weill Cornell Medical College-Qatar, Stem Cell and Microenvironment Laboratory, Education City, Qatar Foundation, Doha 24144, Qatar 8Bioengineering Program, Hofstra University, Hempstead, NY 11549, USA *Correspondence: srafi[email protected] http://dx.doi.org/10.1016/j.devcel.2013.06.017 SUMMARY been appreciated. Capillary ECs of the blood brain barrier (BBB) form a restrictive environment for passage between the Microvascular endothelial cells (ECs) within different brain tissue and the circulating blood. Many of the trafficking pro- tissues are endowed with distinct but as yet unrecog- cesses that are passive in other vascular beds are tightly nized structural, phenotypic, and functional attri- controlled in the brain (Rubin and Staddon, 1999). As opposed butes. We devised EC purification, cultivation, to the BBB, the capillary ECs of the kidney glomeruli are fenes- profiling, and transplantation models that establish trated for the filtration of the blood (Churg and Grishman, tissue-specific molecular libraries of ECs devoid of 1975). -

Vocabulario De Morfoloxía, Anatomía E Citoloxía Veterinaria
Vocabulario de Morfoloxía, anatomía e citoloxía veterinaria (galego-español-inglés) Servizo de Normalización Lingüística Universidade de Santiago de Compostela COLECCIÓN VOCABULARIOS TEMÁTICOS N.º 4 SERVIZO DE NORMALIZACIÓN LINGÜÍSTICA Vocabulario de Morfoloxía, anatomía e citoloxía veterinaria (galego-español-inglés) 2008 UNIVERSIDADE DE SANTIAGO DE COMPOSTELA VOCABULARIO de morfoloxía, anatomía e citoloxía veterinaria : (galego-español- inglés) / coordinador Xusto A. Rodríguez Río, Servizo de Normalización Lingüística ; autores Matilde Lombardero Fernández ... [et al.]. – Santiago de Compostela : Universidade de Santiago de Compostela, Servizo de Publicacións e Intercambio Científico, 2008. – 369 p. ; 21 cm. – (Vocabularios temáticos ; 4). - D.L. C 2458-2008. – ISBN 978-84-9887-018-3 1.Medicina �������������������������������������������������������������������������veterinaria-Diccionarios�������������������������������������������������. 2.Galego (Lingua)-Glosarios, vocabularios, etc. políglotas. I.Lombardero Fernández, Matilde. II.Rodríguez Rio, Xusto A. coord. III. Universidade de Santiago de Compostela. Servizo de Normalización Lingüística, coord. IV.Universidade de Santiago de Compostela. Servizo de Publicacións e Intercambio Científico, ed. V.Serie. 591.4(038)=699=60=20 Coordinador Xusto A. Rodríguez Río (Área de Terminoloxía. Servizo de Normalización Lingüística. Universidade de Santiago de Compostela) Autoras/res Matilde Lombardero Fernández (doutora en Veterinaria e profesora do Departamento de Anatomía e Produción Animal. -

Blood and Lymph Vascular Systems
BLOOD AND LYMPH VASCULAR SYSTEMS BLOOD TRANSFUSIONS Objectives Functions of vessels Layers in vascular walls Classification of vessels Components of vascular walls Control of blood flow in microvasculature Variation in microvasculature Blood barriers Lymphatic system Introduction Multicellular Organisms Need 3 Mechanisms --------------------------------------------------------------- 1. Distribute oxygen, nutrients, and hormones CARDIOVASCULAR SYSTEM 2. Collect waste 3. Transport waste to excretory organs CARDIOVASCULAR SYSTEM Cardiovascular System Component function Heart - Produce blood pressure (systole) Elastic arteries - Conduct blood and maintain pressure during diastole Muscular arteries - Distribute blood, maintain pressure Arterioles - Peripheral resistance and distribute blood Capillaries - Exchange nutrients and waste Venules - Collect blood from capillaries (Edema) Veins - Transmit blood to large veins Reservoir Larger veins - receive lymph and return blood to Heart, blood reservoir Cardiovascular System Heart produces blood pressure (systole) ARTERIOLES – PERIPHERAL RESISTANCE Vessels are structurally adapted to physical and metabolic requirements. Vessels are structurally adapted to physical and metabolic requirements. Cardiovascular System Elastic arteries- conduct blood and maintain pressure during diastole Cardiovascular System Muscular Arteries - distribute blood, maintain pressure Arterioles - peripheral resistance and distribute blood Capillaries - exchange nutrients and waste Venules - collect blood from capillaries -
Why Is Plasma Renin Activity Lower in Populations of African Origin?
Journal of Human Hypertension (2001) 15, 17–25 2001 Macmillan Publishers Ltd All rights reserved 0950-9240/01 $15.00 www.nature.com/jhh REVIEW ARTICLE Why is plasma renin activity lower in populations of African origin? GA Sagnella Blood Pressure Unit, St George’s Hospital Medical School, Cranmer Terrace, London SW17 ORE, UK Plasma renin activity is significantly lower in black the molecular level suggests that the lower PRA may people compared with whites independent of age and arise from gene variation in the renal epithelial sodium blood pressure status. The lower PRA appears to be due channel. The functional significance of the lower PRA in to a reduction in the rate of secretion of renin but the relation to the different pattern of cardiovascular and exact mechanistic events underlying such differences in renal disease between blacks and whites remains renin release between blacks and whites are still not unclear. Moreover, direct investigations of pre-treat- fully understood. Nevertheless, given the paramount ment renin status in hypertensive blacks in relation to importance of the renin-angiotensin system in the con- blood pressure response have demonstrated that the trol of sodium balance, a most likely explanation is that pre-treatment PRA is not a good index of subsequent the lower renin is a consequence of differences in renal blood pressure response to pharmacological treatment. sodium handling between blacks and whites. The lower Nevertheless, the blood pressure reduction to short PRA does not reflect differences in dietary sodium term sodium restriction is greater in blacks compared intake but the evidence available suggests that the low with whites and, in the black subjects, the greater PRA could be part of the corrective mechanisms reduction in blood pressure to sodium restriction designed to maintain sodium balance in the presence appears to be related, at least in part, to the decreased of an increased tendency for sodium retention in black responsiveness of the renin-angiotensin system. -

Vessels and Circulation
CARDIOVASCULAR SYSTEM OUTLINE 23.1 Anatomy of Blood Vessels 684 23.1a Blood Vessel Tunics 684 23.1b Arteries 685 23.1c Capillaries 688 23 23.1d Veins 689 23.2 Blood Pressure 691 23.3 Systemic Circulation 692 Vessels and 23.3a General Arterial Flow Out of the Heart 693 23.3b General Venous Return to the Heart 693 23.3c Blood Flow Through the Head and Neck 693 23.3d Blood Flow Through the Thoracic and Abdominal Walls 697 23.3e Blood Flow Through the Thoracic Organs 700 Circulation 23.3f Blood Flow Through the Gastrointestinal Tract 701 23.3g Blood Flow Through the Posterior Abdominal Organs, Pelvis, and Perineum 705 23.3h Blood Flow Through the Upper Limb 705 23.3i Blood Flow Through the Lower Limb 709 23.4 Pulmonary Circulation 712 23.5 Review of Heart, Systemic, and Pulmonary Circulation 714 23.6 Aging and the Cardiovascular System 715 23.7 Blood Vessel Development 716 23.7a Artery Development 716 23.7b Vein Development 717 23.7c Comparison of Fetal and Postnatal Circulation 718 MODULE 9: CARDIOVASCULAR SYSTEM mck78097_ch23_683-723.indd 683 2/14/11 4:31 PM 684 Chapter Twenty-Three Vessels and Circulation lood vessels are analogous to highways—they are an efficient larger as they merge and come closer to the heart. The site where B mode of transport for oxygen, carbon dioxide, nutrients, hor- two or more arteries (or two or more veins) converge to supply the mones, and waste products to and from body tissues. The heart is same body region is called an anastomosis (ă-nas ′tō -mō′ sis; pl., the mechanical pump that propels the blood through the vessels. -

Arteries.Pdf
Arteries Although blood vessels differ in size, distribution, and function, structurally they share many common features. As in the heart, the walls of blood vessels consist of three major coats or tunics. Differences in the appearance and functions of the various parts of the circulatory system are reflected by structural changes in these tunics or by reduction and even omission of some of the layers. From the lumen outward, the wall of a blood vessel consists of a tunica intima, tunica media, and tunica adventitia. The tunica intima corresponds to and is continuous with the endocardium of the heart. It consists of an endothelium of flattened squamous cells resting on a basal lamina and is supported by a subendothelial connective tissue. The tunica media is the equivalent of the myocardium of the heart and is the layer most variable both in size and structure. Depending on the function of the vessel, this layer contains variable amounts of smooth muscle and elastic tissue. The tunica adventitia also varies in thickness in different parts of the vascular circuit. It consists mainly of collagenous connective tissue and corresponds to the epicardium of the heart, but it lacks mesothelial cells. As arteries course away from the heart they undergo successive divisions to provide numerous branches whose calibers progressively decrease. The changes in size and the corresponding changes in structure of the vessel wall are continuous, but three classes of arteries can be distinguished: large elastic or conducting arteries, medium-sized muscular or distributing arteries, and small arteries and arterioles. A characteristic feature of the entire arterial side of the blood vasculature system is the prominence of smooth muscle in the tunica media. -
Cardiovascular System Summary Notes the Cardiovascular System
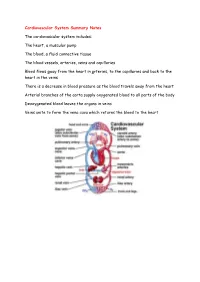
Cardiovascular System Summary Notes The cardiovascular system includes: The heart, a muscular pump The blood, a fluid connective tissue The blood vessels, arteries, veins and capillaries Blood flows away from the heart in arteries, to the capillaries and back to the heart in the veins There is a decrease in blood pressure as the blood travels away from the heart Arterial branches of the aorta supply oxygenated blood to all parts of the body Deoxygenated blood leaves the organs in veins Veins unite to form the vena cava which returns the blood to the heart Pulmonary System This is the route by which blood is circulated from the heart to the lungs and back to the heart again The pulmonary system is exceptional in that the pulmonary artery carries deoxygenated blood and the pulmonary vein carries oxygenated blood Hepatic Portal Vein There is another exception in the circulatory system – the hepatic portal vein Veins normally carry blood from an organ back to the heart The hepatic portal vein carries blood from the capillary bed of the intestine to the capillary bed of the liver As a result, the liver has three blood vessels associated with it Arteries and Veins The central cavity of a blood vessel is called the lumen The lumen is lined with a thin layer of cells called the endothelium The composition of the vessel wall surrounding the endothelium is different in arteries, veins and capillaries Arteries carry blood away from the heart Arteries have a thick middle layer of smooth muscle They have an inner and outer layer of elastic fibres Elastic -

Gli1+ Pericyte Loss Induces Capillary Rarefaction and Proximal Tubular Injury
BRIEF COMMUNICATION www.jasn.org Gli1+ Pericyte Loss Induces Capillary Rarefaction and Proximal Tubular Injury † ‡ ‡ ‡ Rafael Kramann,* Janewit Wongboonsin, § Monica Chang-Panesso, Flavia G. Machado, and ‡ Benjamin D. Humphreys *Renal Division, Brigham and Women’s Hospital, Department of Medicine, Harvard Medical School, Boston, Massachusetts; †Division of Nephrology and Clinical Immunology, RWTH Aachen University Medical Faculty, RWTH Aachen University, Aachen, Germany; ‡Division of Nephrology, Department of Medicine, Washington University School of Medicine in St. Louis, St. Louis, Missouri; and §Department of Medicine, Siriraj Hospital, Mahidol University, Bangkok, Thailand ABSTRACT Peritubular capillary rarefaction is hypothesized to contribute to the increased risk of after AKI, and whether pericyte loss is future CKD after AKI. Here, we directly tested the role of Gli1+ kidney pericytes in the sufficient to induce peritubular capillary maintenance of peritubular capillary health, and the consequences of pericyte loss loss and altered morphology. during injury. Using bigenic Gli1-CreERt2; R26tdTomato reporter mice, we observed We genetically labeled pericytes using increased distance between Gli1+ pericytes and endothelial cells after AKI (mean6 Gli1-CreERt2 mice crossed against SEM: 3.360.1 mm before injury versus 12.560.2 mm after injury; P,0.001). Using a the R26tdTomato reporter mouse (Gt genetic ablation model, we asked whether pericyte loss alone is sufficient for capillary (ROSA)26Sortm9(CAF-tdTomato)Hze/J; destabilization. Ten days after pericyte ablation, we observed endothelial cell damage Figure 1A). After tamoxifen injection, mice by electron microscopy. Furthermore, pericyte loss led to significantly reduced capil- were subjected to severe unilateral ische- lary number at later time points (mean6SEM capillaries/high-power field: 67.664.7 in mia reperfusion injury (IRI; 28-minute control versus 44.164.8 at 56 days; P,0.05) and increased cross-sectional area (mean6 clamp). -

27 Lymph Vascular System
Lymph Vascular System The lymph vascular system consists of endothelial-lined tubes that recover intercellular (tissue) fluid not picked up by the blood vascular system and returns it to the blood. The fluid (lymph) carried by the lymphatics is a blood filtrate formed as fluid crosses the blood capillaries into the tissues. Unlike the blood vascular system, lymph flow is unidirectional - from tissues to the union of the lymphatic and blood vascular systems at the base of the neck. The lymphatic vascular system begins in the tissues as blindly ending capillaries that drain into larger collecting vessels and then into two main lymphatic trunks. Lymph nodes occur along the course of the vessels and filter the lymph. Lymphatics are present in most tissues but are absent from bone marrow, the central nervous system, coats of the eye, internal ear, and fetal placenta. Lymph Capillaries Lymph capillaries are thin-walled, blind tubes that branch and anastomose freely to form a rich network in organs and tissues. They are wider and more irregular than blood capillaries. The wall of a lymph capillary consists only of a thin continuous endothelium and a discontinuous basal lamina that is present only in patches or may even be absent. Adjacent endothelial cells may overlap, but junctional complexes are few and clefts occur between the cells. Externally, the endothelium is surrounded by a small amount of collagenous connective tissue. Fine filaments run perpendicularly from the collagen bundles and attach to the outer surfaces of the endothelium as anchoring filaments that maintain the patency of the vessel. Collecting Lymph Vessels Collecting lymph vessels differ from lymph capillaries in size and the thickness of their walls. -

Terminology Resource File
Terminology Resource File Version 2 July 2012 1 Terminology Resource File This resource file has been compiled and designed by the Northern Assistant Transfusion Practitioner group which was formed in 2008 and who later identified the need for such a file. This resource file is aimed at Assistant Transfusion Practitioners to help them understand the medical terminology and its relevance which they may encounter in the patient’s medical and nursing notes. The resource file will not include all medical complaints or illnesses but will incorporate those which will need to be considered and appreciated if a blood component was to be administered. The authors have taken great care to ensure that the information contained in this document is accurate and up to date. Authors: Jackie Cawthray Carron Fogg Julia Llewellyn Gillian McAnaney Lorna Panter Marsha Whittam Edited by: Denise Watson Document administrator: Janice Robertson ACKNOWLEDGMENTS We would like to acknowledge the following people for providing their valuable feedback on this first edition: Tony Davies Transfusion Liaison Practitioner Rose Gill Transfusion Practitioner Marie Green Transfusion Practitioner Tina Ivel Transfusion Practitioner Terry Perry Transfusion Specialist Janet Ryan Transfusion Practitioner Dr. Hazel Tinegate Consultant Haematologist Reviewed July 2012 Next review due July 2013 Version 2 July 2012 2 Contents Page no. Abbreviation list 6 Abdominal Aortic Aneurysm (AAA) 7 Acidosis 7 Activated Partial Thromboplastin Time (APTT) 7 Acquired Immune Deficiency Syndrome