Clark, J. W., & Donoghue, P. C. J. (2017). Constraining the Timing Of
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Papers in Press
Papers in Press “Papers in Press” includes peer-reviewed, accepted manuscripts of research articles, reviews, and short notes to be published in Paleontological Research. They have not yet been copy edited and/or formatted in the publication style of Paleontological Research. As soon as they are printed, they will be removed from this website. Please note they can be cited using the year of online publication and the DOI, as follows: Humblet, M. and Iryu, Y. 2014: Pleistocene coral assemblages on Irabu-jima, South Ryukyu Islands, Japan. Paleontological Research, doi: 10.2517/2014PR020. doi:10.2517/2018PR013 Features and paleoecological significance of the shark fauna from the Upper Cretaceous Hinoshima Formation, Himenoura Group, Southwest Japan Accepted Naoshi Kitamura 4-8-7 Motoyama, Chuo-ku Kumamoto, Kumamoto 860-0821, Japan (e-mail: [email protected]) Abstract. The shark fauna of the Upper Cretaceous Hinoshima Formation (Santonian: 86.3–83.6 Ma) of the manuscriptHimenoura Group (Kamiamakusa, Kumamoto Prefecture, Kyushu, Japan) was investigated based on fossil shark teeth found at five localities: Himedo Park, Kugushima, Wadanohana, Higashiura, and Kotorigoe. A detailed geological survey and taxonomic analysis was undertaken, and the habitat, depositional environment, and associated mollusks of each locality were considered in the context of previous studies. Twenty-one species, 15 genera, 11 families, and 6 orders of fossil sharks are recognized from the localities. This assemblage is more diverse than has previously been reported for Japan, and Lamniformes and Hexanchiformes were abundant. Three categories of shark fauna are recognized: a coastal region (Himedo Park; probably a breeding site), the coast to the open sea (Kugushima and Wadanohana), and bottom-dwelling or near-seafloor fauna (Kugushima, Wadanohana, Higashiura, and Kotorigoe). -

A New Assemblage of Plant Mesofossils (Late Turonian– Middle Santonian; Upper Cretaceous) from the Tamagawa Formation, Kuji Group, in Northeastern Japan
120Paleontological Research, vol. 25, no. 2, pp. 120–126, MasamichiApril 1, 2021 Takahashi et al. © by the Palaeontological Society of Japan doi:10.2517/2020PR015 A new assemblage of plant mesofossils (late Turonian– middle Santonian; Upper Cretaceous) from the Tamagawa Formation, Kuji Group, in northeastern Japan MASAMICHI TAKAHASHI1, PATRICK S. HERENDEEN2, FABIANY HERRERA2, REN HIRAYAMA3, HISAO ANDO4, KAZUHISA SASAKI5 and PETER R. CRANE6,7 1Department of Environmental Sciences, Niigata University, 8050, 2-cho, Ikarashi, Nishi-ku, Niigata 950-2181, Japan (e-mail: [email protected]) 2Chicago Botanic Garden, 1000 Lake Cook Road, Glencoe, Illinois 60022, USA 3School of International Liberal Studies, Waseda University, 1-7-14, Nishiwaseda, Shinjuku-ku, Tokyo 169-0051, Japan 4Department of Earth Sciences, Faculty of Science, Ibaraki University, 2-1-1, Bunkyo, Mito 310-8512, Japan 5Kuji Amber Museum, 19-156-133, Kokujicho, Kuji, Iwate 028-0071, Japan 6Yale School of the Environment, Yale University, 195, Prospect Street, New Haven, Connecticut 06511, USA 7Oak Spring Garden Foundation, 1776, Loughborough Lane, Upperville, Virginia 20184, USA Received November 14, 2019; Revised manuscript accepted March 4, 2020 Abstract. A preliminary description is provided of a new assemblage of small, three-dimensional and char- coalified mesofossils from the Tamagawa Formation (late Turonian–middle Santonian; Upper Cretaceous) of the Kuji Group in northeastern Japan. The new mesofossils yield excellent structural details and include well- preserved circinate shoots of ferns together with conifer leafy-shoots, seeds and probable pollen cones, and vari- ety of angiosperm fruits and seeds, including fruits of Cornales and seeds of Nymphaeales. The new mesofossil assemblage is complementary to the previously published macrofossil flora from the Kuji Group. -

Earliest Record of Megaphylls and Leafy Structures, and Their Initial Diversification
Review Geology August 2013 Vol.58 No.23: 27842793 doi: 10.1007/s11434-013-5799-x Earliest record of megaphylls and leafy structures, and their initial diversification HAO ShouGang* & XUE JinZhuang Key Laboratory of Orogenic Belts and Crustal Evolution, School of Earth and Space Sciences, Peking University, Beijing 100871, China Received January 14, 2013; accepted February 26, 2013; published online April 10, 2013 Evolutionary changes in the structure of leaves have had far-reaching effects on the anatomy and physiology of vascular plants, resulting in morphological diversity and species expansion. People have long been interested in the question of the nature of the morphology of early leaves and how they were attained. At least five lineages of euphyllophytes can be recognized among the Early Devonian fossil plants (Pragian age, ca. 410 Ma ago) of South China. Their different leaf precursors or “branch-leaf com- plexes” are believed to foreshadow true megaphylls with different venation patterns and configurations, indicating that multiple origins of megaphylls had occurred by the Early Devonian, much earlier than has previously been recognized. In addition to megaphylls in euphyllophytes, the laminate leaf-like appendages (sporophylls or bracts) occurred independently in several dis- tantly related Early Devonian plant lineages, probably as a response to ecological factors such as high atmospheric CO2 concen- trations. This is a typical example of convergent evolution in early plants. Early Devonian, euphyllophyte, megaphyll, leaf-like appendage, branch-leaf complex Citation: Hao S G, Xue J Z. Earliest record of megaphylls and leafy structures, and their initial diversification. Chin Sci Bull, 2013, 58: 27842793, doi: 10.1007/s11434- 013-5799-x The origin and evolution of leaves in vascular plants was phology and evolutionary diversification of early leaves of one of the most important evolutionary events affecting the basal euphyllophytes remain enigmatic. -

Titanosauriform Teeth from the Cretaceous of Japan
“main” — 2011/2/10 — 15:59 — page 247 — #1 Anais da Academia Brasileira de Ciências (2011) 83(1): 247-265 (Annals of the Brazilian Academy of Sciences) Printed version ISSN 0001-3765 / Online version ISSN 1678-2690 www.scielo.br/aabc Titanosauriform teeth from the Cretaceous of Japan HARUO SAEGUSA1 and YUKIMITSU TOMIDA2 1Museum of Nature and Human Activities, Hyogo, Yayoigaoka 6, Sanda, 669-1546, Japan 2National Museum of Nature and Science, 3-23-1 Hyakunin-cho, Shinjuku-ku, Tokyo 169-0073, Japan Manuscript received on October 25, 2010; accepted for publication on January 7, 2011 ABSTRACT Sauropod teeth from six localities in Japan were reexamined. Basal titanosauriforms were present in Japan during the Early Cretaceous before Aptian, and there is the possibility that the Brachiosauridae may have been included. Basal titanosauriforms with peg-like teeth were present during the “mid” Cretaceous, while the Titanosauria with peg-like teeth was present during the middle of Late Cretaceous. Recent excavations of Cretaceous sauropods in Asia showed that multiple lineages of sauropods lived throughout the Cretaceous in Asia. Japanese fossil records of sauropods are conformable with this hypothesis. Key words: Sauropod, Titanosauriforms, tooth, Cretaceous, Japan. INTRODUCTION humerus from the Upper Cretaceous Miyako Group at Moshi, Iwaizumi Town, Iwate Pref. (Hasegawa et al. Although more than twenty four dinosaur fossil local- 1991), all other localities provided fossil teeth (Tomida ities have been known in Japan (Azuma and Tomida et al. 2001, Tomida and Tsumura 2006, Saegusa et al. 1998, Kobayashi et al. 2006, Saegusa et al. 2008, Ohara 2008, Azuma and Shibata 2010). -

Aquatic and Wet Marchantiophyta, Order Metzgeriales: Aneuraceae
Glime, J. M. 2021. Aquatic and Wet Marchantiophyta, Order Metzgeriales: Aneuraceae. Chapt. 1-11. In: Glime, J. M. Bryophyte 1-11-1 Ecology. Volume 4. Habitat and Role. Ebook sponsored by Michigan Technological University and the International Association of Bryologists. Last updated 11 April 2021 and available at <http://digitalcommons.mtu.edu/bryophyte-ecology/>. CHAPTER 1-11: AQUATIC AND WET MARCHANTIOPHYTA, ORDER METZGERIALES: ANEURACEAE TABLE OF CONTENTS SUBCLASS METZGERIIDAE ........................................................................................................................................... 1-11-2 Order Metzgeriales............................................................................................................................................................... 1-11-2 Aneuraceae ................................................................................................................................................................... 1-11-2 Aneura .......................................................................................................................................................................... 1-11-2 Aneura maxima ............................................................................................................................................................ 1-11-2 Aneura mirabilis .......................................................................................................................................................... 1-11-7 Aneura pinguis .......................................................................................................................................................... -

Anatomy of the Late Devonian Sphenopsid Rotafolia Songziensis
Anatomy of the Late Devonian Sphenopsid Rotafolia songziensis , with a Discussion of Stelar Architecture of the Sphenophyllales Author(s): De‐Ming Wang, Shou‐Gang Hao, Qi Wang, and Jin‐Zhuang Xue Source: International Journal of Plant Sciences, Vol. 167, No. 2 (March 2006), pp. 373-383 Published by: The University of Chicago Press Stable URL: http://www.jstor.org/stable/10.1086/499115 . Accessed: 02/04/2015 03:18 Your use of the JSTOR archive indicates your acceptance of the Terms & Conditions of Use, available at . http://www.jstor.org/page/info/about/policies/terms.jsp . JSTOR is a not-for-profit service that helps scholars, researchers, and students discover, use, and build upon a wide range of content in a trusted digital archive. We use information technology and tools to increase productivity and facilitate new forms of scholarship. For more information about JSTOR, please contact [email protected]. The University of Chicago Press is collaborating with JSTOR to digitize, preserve and extend access to International Journal of Plant Sciences. http://www.jstor.org This content downloaded from 159.226.100.224 on Thu, 2 Apr 2015 03:18:06 AM All use subject to JSTOR Terms and Conditions Int. J. Plant Sci. 167(2):373–383. 2006. Ó 2006 by The University of Chicago. All rights reserved. 1058-5893/2006/16702-0020$15.00 ANATOMY OF THE LATE DEVONIAN SPHENOPSID ROTAFOLIA SONGZIENSIS, WITH A DISCUSSION OF STELAR ARCHITECTURE OF THE SPHENOPHYLLALES De-Ming Wang,*,y Shou-Gang Hao,1,* Qi Wang,z and Jin-Zhuang Xue* *Key Laboratory of Orogenic Belts and Crustal Evolution, Department of Geology, Peking University, Beijing 100871, China; yInstitute for Earth Sciences, University of Graz, A-8010 Graz, Austria; and zKey Laboratory of Systematic and Evolutionary Botany, Institute of Botany, Chinese Academy of Sciences, Beijing 100093, China A previous study of the Late Devonian (Famennian) sphenopsid Rotafolia songziensis Wang, Hao, and Wang provided detailed descriptions of the morphology and a sketchy illustration of a three-ribbed primary xylem. -

Deep Sea Drilling Project Initial Reports Volume 6
39. PLANKTONIC MICROFOSSIL BIOSTRATIGRAPHY OF THE NORTHWESTERN PACIFIC OCEAN David Bukry1, U.S. Geological Survey, La Jolla, California, Robert G. Douglas, Case Western Reserve University, Cleveland, Ohio, Stanley A. Kling, Cities Service Oil Company, Tulsa, Oklahoma, and Valeri Krasheninnikov, Academy of Sciences of the U.S.S.R., Moscow CONTENTS Page Page Introduction 1253 Regional Correlation 1281 Zonal Comparison 1254 Calcareous Nannoplankton 1281 Planktonic Foraminifera, Mesozoic 1281 Upper Cretaceous-Paleocene Boundary 1255 California 1281 Paleocene-Eocene Boundary 1259 Japan 1285 Eocene-Oligocene Boundary 1259 West Pacific 1286 Oligocene-Miocene Boundary 1259 Australia 1287 Miocene-Pliocene Boundary 1260 Planktonic Foraminifera, Cenozoic 1288 Pliocene-Pleistocene Boundary 1261 Solomon Islands 1288 Zonal Summary 1261 Mariana Islands 1288 The Philippines 1288 Paleoecology 1261 Taiwan 1289 Calcareous Nannoplankton 1261 Japan 1289 Radiolaria 1267 Kamchatka Penisula 1290 California 1290 Preservation 1267 Radiolaria 1291 Calcareous Nannoplankton 1267 Sedimentation Rates 1291 Foraminifera 1269 Relationship to Acoustostratigraphy 1294 Radiolaria 1279 References 1296 INTRODUCTION A comparison of zonal units of calcareous nannoplank- ton, foraminifera, and radiolarians in the same strata Biostratigraphic evidence obtained from the north- shows only few cases of exact coincidence of zonal western Pacific Ocean as a result of coring carried out limits, especially if coincidences at the top or bottom by the Glomar Challenger during Leg 6 of the Deep of the standard 9-meter coring runs are dismissed as Sea Drilling Project from Hawaii to Guam is considered artificially induced owing to gaps in sediment recovery. here mainly from the standpoint of three dominant Exact coincidence of zonal limits within coring runs marine planktonic microfossil groups—calcareous nan- are most notable for the Upper Paleocene sediment of noplankton, foraminifers, and radiolarians. -

A Taxonomic Revision of Aneuraceae (Marchantiophyta) from Eastern Africa with an Interactive Identification Key
cryptogamie Bryologie 2019 ● 41 ● 2 DIRECTEUR DE LA PUBLICATION : Bruno David, Président du Muséum national d’Histoire naturelle RÉDACTEURS EN CHEF / EDITORS-IN-CHIEF : Denis LAMY, Michelle Price ASSISTANTS DE RÉDACTION / ASSISTANT EDITORS : Marianne SALAÜN ([email protected]) MISE EN PAGE / PAGE LAYOUT : Marianne SALAÜN RÉDACTEURS ASSOCIÉS / ASSOCIATE EDITORS Biologie moléculaire et phylogénie / Molecular biology and phylogeny Bernard GOFFINET Department of Ecology and Evolutionary Biology, University of Connecticut (United States) Mousses d’Europe / European mosses Isabel DRAPER Centro de Investigación en Biodiversidad y Cambio Global (CIBC-UAM), Universidad Autónoma de Madrid (Spain) Francisco LARA GARCÍA Centro de Investigación en Biodiversidad y Cambio Global (CIBC-UAM), Universidad Autónoma de Madrid (Spain) Mousses d’Afrique et d’Antarctique / African and Antarctic mosses Rysiek OCHYRA Laboratory of Bryology, Institute of Botany, Polish Academy of Sciences, Krakow (Pologne) Bryophytes d’Asie / Asian bryophytes Rui-Liang ZHU School of Life Science, East China Normal University, Shanghai (China) Bioindication / Biomonitoring Franck-Olivier DENAYER Faculté des Sciences Pharmaceutiques et Biologiques de Lille, Laboratoire de Botanique et de Cryptogamie, Lille (France) Écologie des bryophytes / Ecology of bryophyte Nagore GARCÍA MEDINA Department of Biology (Botany), and Centro de Investigación en Biodiversidad y Cambio Global (CIBC-UAM), Universidad Autónoma de Madrid (Spain) COUVERTURE / COVER : From top left, to bottom right, by -

Ecological Sorting of Vascular Plant Classes During the Paleozoic Evolutionary Radiation
i1 Ecological Sorting of Vascular Plant Classes During the Paleozoic Evolutionary Radiation William A. DiMichele, William E. Stein, and Richard M. Bateman DiMichele, W.A., Stein, W.E., and Bateman, R.M. 2001. Ecological sorting of vascular plant classes during the Paleozoic evolutionary radiation. In: W.D. Allmon and D.J. Bottjer, eds. Evolutionary Paleoecology: The Ecological Context of Macroevolutionary Change. Columbia University Press, New York. pp. 285-335 THE DISTINCTIVE BODY PLANS of vascular plants (lycopsids, ferns, sphenopsids, seed plants), corresponding roughly to traditional Linnean classes, originated in a radiation that began in the late Middle Devonian and ended in the Early Carboniferous. This relatively brief radiation followed a long period in the Silurian and Early Devonian during wrhich morphological complexity accrued slowly and preceded evolutionary diversifications con- fined within major body-plan themes during the Carboniferous. During the Middle Devonian-Early Carboniferous morphological radiation, the major class-level clades also became differentiated ecologically: Lycopsids were cen- tered in wetlands, seed plants in terra firma environments, sphenopsids in aggradational habitats, and ferns in disturbed environments. The strong con- gruence of phylogenetic pattern, morphological differentiation, and clade- level ecological distributions characterizes plant ecological and evolutionary dynamics throughout much of the late Paleozoic. In this study, we explore the phylogenetic relationships and realized ecomorphospace of reconstructed whole plants (or composite whole plants), representing each of the major body-plan clades, and examine the degree of overlap of these patterns with each other and with patterns of environmental distribution. We conclude that 285 286 EVOLUTIONARY PALEOECOLOGY ecological incumbency was a major factor circumscribing and channeling the course of early diversification events: events that profoundly affected the structure and composition of modern plant communities. -

Wikstrom2009chap13.Pdf
Liverworts (Marchantiophyta) Niklas Wikströma,*, Xiaolan He-Nygrénb, and our understanding of phylogenetic relationships among A. Jonathan Shawc major lineages and the origin and divergence times of aDepartment of Systematic Botany, Evolutionary Biology Centre, those lineages. Norbyvägen 18D, Uppsala University, Norbyvägen 18D 75236, Altogether, liverworts (Phylum Marchantiophyta) b Uppsala, Sweden; Botanical Museum, Finnish Museum of Natural comprise an estimated 5000–8000 living species (8, 9). History, University of Helsinki, P.O. Box 7, 00014 Helsinki, Finland; Early and alternative classiA cations for these taxa have cDepartment of Biology, Duke University, Durham, NC 27708, USA *To whom correspondence should be addressed (niklas.wikstrom@ been numerous [reviewed by Schuster ( 10)], but the ebc.uu.se) arrangement of terminal taxa (species, genera) into lar- ger groups (e.g., families and orders) based on morpho- logical criteria alone began in the 1960s and 1970s with Abstract the work of Schuster (8, 10, 11) and Schljakov (12, 13), and culminated by the turn of the millenium with the work Liverworts (Phylum Marchantiophyta) include 5000–8000 of Crandall-Stotler and Stotler (14). 7 ree morphological species. Phylogenetic analyses divide liverworts into types of plant bodies (gametophytes) have generally been Haplomitriopsida, Marchantiopsida, and Jungerman- recognized and used in liverwort classiA cations: “com- niopsida. Complex thalloids are grouped with Blasiales in plex thalloids” including ~6% of extant species diversity Marchantiopsida, and leafy liverworts are grouped with and with a thalloid gametophyte that is organized into Metzgeriidae and Pelliidae in Jungermanniopsida. The distinct layers; “leafy liverworts”, by far the most speci- timetree shows an early Devonian (408 million years ago, ose group, including ~86% of extant species diversity and Ma) origin for extant liverworts. -
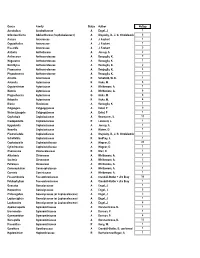
Family Status Author Nospp Acrobolbus Acrobolbaceae a Engel, J
Genus Family Status Author NoSpp Acrobolbus Acrobolbaceae A Engel, J. 1 Odontoschisma Adelanthaceae (Cephaloziaceae?) A Krayesky, D., J. G. Chmielewski 6 Aneura Aneuraceae A J. Faubert 1 Cryptothallus Aneuraceae A J. Faubert 1 Riccardia Aneuraceae A J. Faubert 5 Anthelia Antheliaceae A Jessup, S. 2 Anthoceros Anthocerotaceae A Renzaglia, K. 7 Megaceros Anthocerotaceae A Renzaglia, K. 1 Notothylas Anthocerotaceae A Renzaglia, K. 2 Phaeoceros Anthocerotaceae A Renzaglia, K. 4 Phymatoceros Anthocerotaceae A Renzaglia, K. 1 Arnellia Arnelliaceae R Schofield, W. B. 1 Asterella Aytoniaceae R Hicks, M. 8 Cryptomitrium Aytoniaceae A Whittemore, A. 1 Mannia Aytoniaceae A Whittemore, A. 6 Plagiochasma Aytoniaceae G Hicks, M. 6 Reboulia Aytoniaceae R Hicks, M. 4 Blasia Blasiaceae A Renzaglia, K. 1 Calypogeia Calypogejaceae A Eckel, P 9 Metacalypogeia Calypogejaceae A Eckel, P 1 Cephalozia Cephaloziaceae A Newmaster, S. 11 Cladopodiella Cephaloziaceae R Leonardi, L. 1 Hygrobiella Cephaloziaceae A Jessup, S. 1 Nowellia Cephaloziaceae A Waters, D. 1 Pleurocladula Cephaloziaceae A Krayesky, D., J. G. Chmielewski 1 Schofieldia Cephaloziaceae R Godfrey, J. 1 Cephaloziella Cephaloziellaceae A Wagner, D. 21 Cylindrocolea Cephaloziellaceae A Wagner, D. 1 Chonecolea Chonecoleaceae R Breil, D. 1 Athalamia Cleveaceae A Whittemore, A. 1 Sauteria Cleveaceae A Whittemore, A. 2 Peltolepis Cleveaceae A Whittemore, A. 1 Conocephalum Conocephalaceae A Whittemore, A. 1 Corsinia Corsiniaceae A Whittemore, A. 1 Fossombronia Fossombroniaceae A Crandall-Stotler + Jim Bray 10 Petalophyllum Fossombroniaceae A Crandall-Stotler + Jim Bray 1 Geocalyx Geocalycaceae A Engel, J. 1 Harpanthus Geocalycaceae A Engel, J. 3 Chiloscyphus Geocalycaceae (or Lophocoleaceae) A Engel, J. 3 Leptoscyphus Geocalycaceae (or Lophocoleaceae) A Engel, J. -

Diversity and Evolution of the Megaphyll in Euphyllophytes
G Model PALEVO-665; No. of Pages 16 ARTICLE IN PRESS C. R. Palevol xxx (2012) xxx–xxx Contents lists available at SciVerse ScienceDirect Comptes Rendus Palevol w ww.sciencedirect.com General palaeontology, systematics and evolution (Palaeobotany) Diversity and evolution of the megaphyll in Euphyllophytes: Phylogenetic hypotheses and the problem of foliar organ definition Diversité et évolution de la mégaphylle chez les Euphyllophytes : hypothèses phylogénétiques et le problème de la définition de l’organe foliaire ∗ Adèle Corvez , Véronique Barriel , Jean-Yves Dubuisson UMR 7207 CNRS-MNHN-UPMC, centre de recherches en paléobiodiversité et paléoenvironnements, 57, rue Cuvier, CP 48, 75005 Paris, France a r t i c l e i n f o a b s t r a c t Article history: Recent paleobotanical studies suggest that megaphylls evolved several times in land plant st Received 1 February 2012 evolution, implying that behind the single word “megaphyll” are hidden very differ- Accepted after revision 23 May 2012 ent notions and concepts. We therefore review current knowledge about diverse foliar Available online xxx organs and related characters observed in fossil and living plants, using one phylogenetic hypothesis to infer their origins and evolution. Four foliar organs and one lateral axis are Presented by Philippe Taquet described in detail and differ by the different combination of four main characters: lateral organ symmetry, abdaxity, planation and webbing. Phylogenetic analyses show that the Keywords: “true” megaphyll appeared at least twice in Euphyllophytes, and that the history of the Euphyllophytes Megaphyll four main characters is different in each case. The current definition of the megaphyll is questioned; we propose a clear and accurate terminology in order to remove ambiguities Bilateral symmetry Abdaxity of the current vocabulary.