A Taxonomic Revision of Aneuraceae (Marchantiophyta) from Eastern Africa with an Interactive Identification Key
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Aquatic and Wet Marchantiophyta, Order Metzgeriales: Aneuraceae
Glime, J. M. 2021. Aquatic and Wet Marchantiophyta, Order Metzgeriales: Aneuraceae. Chapt. 1-11. In: Glime, J. M. Bryophyte 1-11-1 Ecology. Volume 4. Habitat and Role. Ebook sponsored by Michigan Technological University and the International Association of Bryologists. Last updated 11 April 2021 and available at <http://digitalcommons.mtu.edu/bryophyte-ecology/>. CHAPTER 1-11: AQUATIC AND WET MARCHANTIOPHYTA, ORDER METZGERIALES: ANEURACEAE TABLE OF CONTENTS SUBCLASS METZGERIIDAE ........................................................................................................................................... 1-11-2 Order Metzgeriales............................................................................................................................................................... 1-11-2 Aneuraceae ................................................................................................................................................................... 1-11-2 Aneura .......................................................................................................................................................................... 1-11-2 Aneura maxima ............................................................................................................................................................ 1-11-2 Aneura mirabilis .......................................................................................................................................................... 1-11-7 Aneura pinguis .......................................................................................................................................................... -

Article ISSN 1179-3163 (Online Edition)
Phytotaxa 63: 21–68 (2012) ISSN 1179-3155 (print edition) www.mapress.com/phytotaxa/ PHYTOTAXA Copyright © 2012 Magnolia Press Article ISSN 1179-3163 (online edition) Early Land Plants Today: Index of Liverworts & Hornworts 2009–2010 LARS SÖDERSTRÖM1, ANDERS HAGBORG2, MARSHALL R. CROSBY3 & MATT VON KONRAT2 1 Department of Biology, Norwegian University of Science and Technology, N-7491, Trondheim, Norway; [email protected] 2 Department of Botany, The Field Museum, 1400 South Lake Shore Drive, Chicago, IL 60605–2496, U.S.A.;[email protected], [email protected] 3 Missouri Botanical Garden, P. O. Box 299, St. Louis, MO 63166–0299 U.S.A.; [email protected] Abstract A widely accessible list of known plant species is a fundamental requirement for plant conservation and has vast applications. An index of published names of liverworts and hornworts between 2009 and 2010 is provided as part of a continued effort in working toward producing a world checklist of this group. Included in the list are also names overlooked by earlier indices. The list includes 30 higher taxa, 250 species, 52 infraspecific taxa, 31 autonyms, and two fossils for 2009 and 2010. A number of taxa not covered by the earlier indices for 2000-2008 are also included. Key words: Liverworts, hornworts, index, nomenclature Introduction Under the auspices of the Early Land Plants Today project, there has been a strong community-driven effort attempting to address the critical need to synthesize the vast nomenclatural, taxonomic and global distributional data for liverworts (Marchantiophyta) and hornworts (Anthocerotophyta) (von Konrat et al. 2010a). These endeavours are critical in providing the foundation to develop a working checklist of liverworts and hornworts worldwide; the first version is projected to be published in 2012. -

Wikstrom2009chap13.Pdf
Liverworts (Marchantiophyta) Niklas Wikströma,*, Xiaolan He-Nygrénb, and our understanding of phylogenetic relationships among A. Jonathan Shawc major lineages and the origin and divergence times of aDepartment of Systematic Botany, Evolutionary Biology Centre, those lineages. Norbyvägen 18D, Uppsala University, Norbyvägen 18D 75236, Altogether, liverworts (Phylum Marchantiophyta) b Uppsala, Sweden; Botanical Museum, Finnish Museum of Natural comprise an estimated 5000–8000 living species (8, 9). History, University of Helsinki, P.O. Box 7, 00014 Helsinki, Finland; Early and alternative classiA cations for these taxa have cDepartment of Biology, Duke University, Durham, NC 27708, USA *To whom correspondence should be addressed (niklas.wikstrom@ been numerous [reviewed by Schuster ( 10)], but the ebc.uu.se) arrangement of terminal taxa (species, genera) into lar- ger groups (e.g., families and orders) based on morpho- logical criteria alone began in the 1960s and 1970s with Abstract the work of Schuster (8, 10, 11) and Schljakov (12, 13), and culminated by the turn of the millenium with the work Liverworts (Phylum Marchantiophyta) include 5000–8000 of Crandall-Stotler and Stotler (14). 7 ree morphological species. Phylogenetic analyses divide liverworts into types of plant bodies (gametophytes) have generally been Haplomitriopsida, Marchantiopsida, and Jungerman- recognized and used in liverwort classiA cations: “com- niopsida. Complex thalloids are grouped with Blasiales in plex thalloids” including ~6% of extant species diversity Marchantiopsida, and leafy liverworts are grouped with and with a thalloid gametophyte that is organized into Metzgeriidae and Pelliidae in Jungermanniopsida. The distinct layers; “leafy liverworts”, by far the most speci- timetree shows an early Devonian (408 million years ago, ose group, including ~86% of extant species diversity and Ma) origin for extant liverworts. -
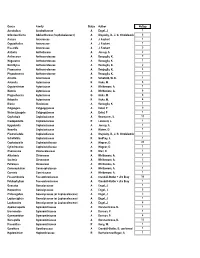
Family Status Author Nospp Acrobolbus Acrobolbaceae a Engel, J
Genus Family Status Author NoSpp Acrobolbus Acrobolbaceae A Engel, J. 1 Odontoschisma Adelanthaceae (Cephaloziaceae?) A Krayesky, D., J. G. Chmielewski 6 Aneura Aneuraceae A J. Faubert 1 Cryptothallus Aneuraceae A J. Faubert 1 Riccardia Aneuraceae A J. Faubert 5 Anthelia Antheliaceae A Jessup, S. 2 Anthoceros Anthocerotaceae A Renzaglia, K. 7 Megaceros Anthocerotaceae A Renzaglia, K. 1 Notothylas Anthocerotaceae A Renzaglia, K. 2 Phaeoceros Anthocerotaceae A Renzaglia, K. 4 Phymatoceros Anthocerotaceae A Renzaglia, K. 1 Arnellia Arnelliaceae R Schofield, W. B. 1 Asterella Aytoniaceae R Hicks, M. 8 Cryptomitrium Aytoniaceae A Whittemore, A. 1 Mannia Aytoniaceae A Whittemore, A. 6 Plagiochasma Aytoniaceae G Hicks, M. 6 Reboulia Aytoniaceae R Hicks, M. 4 Blasia Blasiaceae A Renzaglia, K. 1 Calypogeia Calypogejaceae A Eckel, P 9 Metacalypogeia Calypogejaceae A Eckel, P 1 Cephalozia Cephaloziaceae A Newmaster, S. 11 Cladopodiella Cephaloziaceae R Leonardi, L. 1 Hygrobiella Cephaloziaceae A Jessup, S. 1 Nowellia Cephaloziaceae A Waters, D. 1 Pleurocladula Cephaloziaceae A Krayesky, D., J. G. Chmielewski 1 Schofieldia Cephaloziaceae R Godfrey, J. 1 Cephaloziella Cephaloziellaceae A Wagner, D. 21 Cylindrocolea Cephaloziellaceae A Wagner, D. 1 Chonecolea Chonecoleaceae R Breil, D. 1 Athalamia Cleveaceae A Whittemore, A. 1 Sauteria Cleveaceae A Whittemore, A. 2 Peltolepis Cleveaceae A Whittemore, A. 1 Conocephalum Conocephalaceae A Whittemore, A. 1 Corsinia Corsiniaceae A Whittemore, A. 1 Fossombronia Fossombroniaceae A Crandall-Stotler + Jim Bray 10 Petalophyllum Fossombroniaceae A Crandall-Stotler + Jim Bray 1 Geocalyx Geocalycaceae A Engel, J. 1 Harpanthus Geocalycaceae A Engel, J. 3 Chiloscyphus Geocalycaceae (or Lophocoleaceae) A Engel, J. 3 Leptoscyphus Geocalycaceae (or Lophocoleaceae) A Engel, J. -

Article ISSN 2381-9685 (Online Edition)
Bry. Div. Evo. 043 (1): 284–306 ISSN 2381-9677 (print edition) DIVERSITY & https://www.mapress.com/j/bde BRYOPHYTEEVOLUTION Copyright © 2021 Magnolia Press Article ISSN 2381-9685 (online edition) https://doi.org/10.11646/bde.43.1.20 Advances in understanding of mycorrhizal-like associations in bryophytes SILVIA PRESSEL1*, MARTIN I. BIDARTONDO2, KATIE J. FIELD3 & JEFFREY G. DUCKETT1 1Life Sciences Department, The Natural History Museum, Cromwell Road, London SW7 5BD, UK; �[email protected]; https://orcid.org/0000-0001-9652-6338 �[email protected]; https://orcid.org/0000-0001-7101-6673 2Imperial College London and Royal Botanic Gardens, Kew TW9 3DS, UK; �[email protected]; https://orcid.org/0000-0003-3172-3036 3 Department of Animal and Plant Sciences, University of Sheffield, Sheffield, S10 2TN, UK; �[email protected]; https://orcid.org/0000-0002-5196-2360 * Corresponding author Abstract Mutually beneficial associations between plants and soil fungi, mycorrhizas, are one of the most important terrestrial symbioses. These partnerships are thought to have propelled plant terrestrialisation some 500 million years ago and today they play major roles in ecosystem functioning. It has long been known that bryophytes harbour, in their living tissues, fungal symbionts, recently identified as belonging to the three mycorrhizal fungal lineages Glomeromycotina, Ascomycota and Basidiomycota. Latest advances in understanding of fungal associations in bryophytes have been largely driven by the discovery, nearly a decade ago, that early divergent liverwort clades, including the most basal Haplomitriopsida, and some hornworts, engage with a wider repertoire of fungal symbionts than previously thought, including endogonaceous members of the ancient sub-phylum Mucoromycotina. -

Arctic Biodiversity Assessment
310 Arctic Biodiversity Assessment Purple saxifrage Saxifraga oppositifolia is a very common plant in poorly vegetated areas all over the high Arctic. It even grows on Kaffeklubben Island in N Greenland, at 83°40’ N, the most northerly plant locality in the world. It is one of the first plants to flower in spring and serves as the territorial flower of Nunavut in Canada. Zackenberg 2003. Photo: Erik Thomsen. 311 Chapter 9 Plants Lead Authors Fred J.A. Daniëls, Lynn J. Gillespie and Michel Poulin Contributing Authors Olga M. Afonina, Inger Greve Alsos, Mora Aronsson, Helga Bültmann, Stefanie Ickert-Bond, Nadya A. Konstantinova, Connie Lovejoy, Henry Väre and Kristine Bakke Westergaard Contents Summary ..............................................................312 9.4. Algae ..............................................................339 9.1. Introduction ......................................................313 9.4.1. Major algal groups ..........................................341 9.4.2. Arctic algal taxonomic diversity and regionality ..............342 9.2. Vascular plants ....................................................314 9.4.2.1. Russia ...............................................343 9.2.1. Taxonomic categories and species groups ....................314 9.4.2.2. Svalbard ............................................344 9.2.2. The Arctic territory and its subdivision .......................315 9.4.2.3. Greenland ...........................................344 9.2.3. The flora of the Arctic ........................................316 -

Comparison of Invertebrates and Lichens Between Young and Ancient
Comparison of invertebrates and lichens between young and ancient yew trees Bachelor agro & biotechnology Specialization Green management 3th Internship report / bachelor dissertation Student: Clerckx Jonathan Academic year: 2014-2015 Tutor: Ms. Joos Isabelle Mentor: Ms. Birch Katherine Natural England: Kingley Vale NNR Downs Road PO18 9BN Chichester www.naturalengland.org.uk Comparison of invertebrates and lichens between young and ancient yew trees. Natural England: Kingley Vale NNR Foreword My dissertation project and internship took place in an ancient yew woodland reserve called Kingley Vale National Nature Reserve. Kingley Vale NNR is managed by Natural England. My dissertation deals with the biodiversity in these woodlands. During my stay in England I learned many things about the different aspects of nature conservation in England. First of all I want to thank Katherine Birch (manager of Kingley Vale NNR) for giving guidance through my dissertation project and for creating lots of interesting days during my internship. I want to thank my tutor Isabelle Joos for suggesting Kingley Vale NNR and guiding me during the year. I thank my uncle Guido Bonamie for lending me his microscope and invertebrate books and for helping me with some identifications of invertebrates. I thank Lies Vandercoilden for eliminating my spelling and grammar faults. Thanks to all the people helping with identifications of invertebrates: Guido Bonamie, Jon Webb, Matthew Shepherd, Bryan Goethals. And thanks to the people that reacted on my posts on the Facebook page: Lichens connecting people! I want to thank Catherine Slade and her husband Nigel for being the perfect hosts of my accommodation in England. -

Aneuraceae, Marchantiophytina)
European Journal of Taxonomy 273: 1–26 ISSN 2118-9773 http://dx.doi.org/10.5852/ejt.2017.273 www.europeanjournaloftaxonomy.eu 2017 · Rabeau L. et al. This work is licensed under a Creative Commons Attribution 3.0 License. DNA Library of Life, research article New insights into the phylogeny and relationships within the worldwide genus Riccardia (Aneuraceae, Marchantiophytina) Lucile RABEAU 1,*, S. Robbert GRADSTEIN 2, Jean-Yves DUBUISSON 3, Martin NEBEL 4, Dietmar QUANDT 5 & Catherine REEB 6 1,2,3,6 Université Pierre et Marie Curie – Sorbonne Universités – Institut de Systématique, Évolution, Biodiversité, ISYEB, UMR 7205, CNRS-MNHN-UPMC-EPHE, Muséum national d’Histoire naturelle, 57 rue Cuvier, CP 39, 75005 Paris, France. 4 Staatliches Museum für Naturkunde Stuttgart, Rosenstein 1, 70191 Stuttgart, Germany. 5 Nees-Institut für Biodiversität der Pfl anzen, Rheinische Friedrich-Wilhelms-Universität Bonn, Meckenheimer Allee 170, 53115 Bonn, Germany. * Corresponding author: [email protected] 2 Email: [email protected] 3 Email: [email protected] 4 Email: [email protected] 5 Email: [email protected] 6 Email: [email protected] Abstract. With 280 accepted species, the genus Riccardia S.F.Gray (Aneuraceae) is one of the most speciose genera of simple thalloid liverworts. The current classifi cation of this genus is based on morphological and limited-sampling molecular studies. Very few molecular data are available and a comprehensive view of evolutionary relationships within the genus is still lacking. A phylogeny focusing on relationships within the large genus Riccardia has not been conducted. Here we propose the fi rst worldwide molecular phylogeny of the genus Riccardia, based on Bayesian inference and parsimony ratchet analyses of sequences from three plastid regions (psbA-trnH, rps4, trnL-F). -

Riccardia Regnellii, an Older Name for R. Amazonica (Marchantiophyta
Cryptogamie, Bryologie, 2018, 39 (3): 305-308 © 2018 Adac. Tous droits réservés Riccardia regnellii,anolder name for R. amazonica (Marchantiophyta: Aneuraceae) S. Robbert GRadStein * &Catherine ReeB Muséum national d’Histoire naturelle, institut de Systématique, Évolution, Biodiversité (UMR 7205), Herbier national, CP 39, 12 rue Buffon, 75005 Paris, France Abstract – Riccardia amazonica is one of the most widespread tropical species in the genus Riccardia,occurring on soil, shaded rock and rotten wood in lowland and montane rainforests throughout tropical South America. Astudy of type specimens shows that the name R. amazonica should be replaced by R. regnellii.The latter species has been confused with R. sprucei. Riccardia regnellii is afurther example of a Riccardia species that can be monoicous or dioicous. African specimens identified as R. amazonica do not belong to this species; the correct name for the African plants is R. longispica. Riccardia regnellii is lectotypified. Liverworts /monoicy / Riccardia /synonymy /taxonomy /tropical America /typification INTRODUCTION Riccardia amazonica (Spruce) Gradst. &Hekking is widespread in tropical South America,occurring on soil, shaded rock and decaying wood in lowland and montane rainforests up to the páramo belt (Meenks, 1987; Gradstein &Costa, 2003). The species has additionally been recorded from Africa (Meenks &Pócs, 1985; Perold, 2003) but Reeb &Bardat (2014) found that African specimens did not belong to R. amazonica and questioned the occurrence of the species in Africa (see below). -

Checklist of the Liverworts and Hornworts of New Caledonia1
Cryptogamie, Bryologie, 2011, 32 (4): 287-390 ©2011 Adac. Tous droits réservés Checklist of the liverworts and hornworts of New Caledonia1 Louis THOUVENOT a,S.Robbert GRADSTEIN b,Anders HAGBORG c, Lars SÖDERSTRÖM d &Jacques BARDAT b a11, rue Saint-Léon, 66000 Perpignan, France bMuseum National d’Histoire Naturelle, Département Systématique et Évolution, UMR 7205, CP 39, 57 rue Cuvier, 75231 Paris Cedex 05, France cDepartment of Botany, The Field Museum, 1400 South Lake Shore Drive, Chicago, IL 60605–2496, U.S.A. dDepartment of Biology, Norwegian University of Science and Technology, 7491 Trondheim, Norway Abstract –The present checklist of the liverworts and hornworts of New Caledonia is a sequel to the recent catalogue of the mosses and accepts 464 species and 18 infraspecific taxa, in 104 genera and 39 families. In addition, 32 species are doubtful records of New Caledonia and 52 species are excluded. The hepatic flora of New Caledonia is more similar to that of Indomalesia than of Australasia, which may be explained by the predominantly tropical climate of New Caledonia. The composition of the flora is considered the result of a long history of dispersal and speciation events since 37 Ma. Endemism of liverworts and hornworts of New Caledonia is between 13% (confirmed endemics, treated in recent revision or monographs) and 39% (confirmed and potential endemics). The latter figure is the same as for mosses. One genus of liverworts, Meinungeria (Lepidoziaceae), is endemic to New Caledonia. Cheilolejeunea xanthocarpa, Mastigolejeunea indica and Microlejeunea lunulatiloba are reported as new to New Caledonia. The new name Cololejeunea aurantia (Tixier) Thouvenot comb. -

A Bryophyte Species List for Denali National Park and Preserve, Alaska, with Comments on Several New and Noteworthy Records Author(S): Sarah E
A Bryophyte Species List for Denali National Park and Preserve, Alaska, with Comments on Several New and Noteworthy Records Author(s): Sarah E. Stehn , James K. Walton , Carl A. Roland Source: Evansia, 30(1):31-45. 2013. Published By: The American Bryological and Lichenological Society, Inc. DOI: http://dx.doi.org/10.1639/079.030.0105 URL: http://www.bioone.org/doi/full/10.1639/079.030.0105 BioOne (www.bioone.org) is a nonprofit, online aggregation of core research in the biological, ecological, and environmental sciences. BioOne provides a sustainable online platform for over 170 journals and books published by nonprofit societies, associations, museums, institutions, and presses. Your use of this PDF, the BioOne Web site, and all posted and associated content indicates your acceptance of BioOne’s Terms of Use, available at www.bioone.org/page/ terms_of_use. Usage of BioOne content is strictly limited to personal, educational, and non-commercial use. Commercial inquiries or rights and permissions requests should be directed to the individual publisher as copyright holder. BioOne sees sustainable scholarly publishing as an inherently collaborative enterprise connecting authors, nonprofit publishers, academic institutions, research libraries, and research funders in the common goal of maximizing access to critical research. Evansia 30(1) 31 A bryophyte species list for Denali National Park and Preserve, Alaska, with comments on several new and noteworthy records Sarah E. Stehn Denali National Park and Preserve and Central Alaska Network National Park Service, P.O. Box 9, Denali Park, AK 99755 E-mail: [email protected] James K. Walton Southwest Alaska Network National Park Service, 240 West 5th Avenue, Anchorage, AK 99501 E-mail: [email protected] Carl A. -

(Aneuraceae, Hepaticopsida) in the Iberian Peninsula
J Hallori Bot. Lab. No. 97: 309- 3J6 (Jan. 2(05) MODELLING THE DISTRIBUTION OF CRYPTOTHALLUS MlRABILIS MALMB. (ANEURACEAE, HEPATICOPSIDA) IN THE IBERIAN PENINSULA CECIUA SERGIO', DAVID DRAPER2 AND CE:SAR GARCIA' ABSTRACT . Cryptothallus mirabilis Malmb. exhibits a specialised ecology and is a non-photosyn thetic Iiverwort. This species was first described for Scandinavia and till now was considered a north oceanic species. The discoveries of C. mirabilis in different localities in Portugal are the first reliable records of the species in southern Europe and represent an extension of its geographical range. [n fact, in Portugal C. mirabilis does not seem to be rare in areas with oceanic influence, mainly in wet forestry habitats. It is possibly widespread in the Iberian Peninsula because it is easily overlooked owing to its subterranean growth. The objective of this work is to give new localities which con tribute to an evaluation of its ecological requirements, to the definition of its biogeographical distrib ution in Portugal, and to present a predictive map for the Iberian Peninsula. The aim of the model is to identify the distribution pattern and also some potential places of occurrence in Spain and provide information about the environmental range of the species in order to improve actions for conserva tion. K EY WORDS: Cryptothallus mirabilis, ecology, predictive map, conservation, Iberian Peninsula INTRODUCTION The discoveries of Cryptothallus mirabilis Malmb. in Portugal (Sergio & Seneca 1997, Sergio & Garcia 1999) are the first reliable records of the species in southern Europe and represent an important extension of its geographic range. At that time, owing to our limited experience of the ecology of this species, the dis covery of C.