Saatgut Vom Natürlichen Standort/Seeds From
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Subalpine Dwarf Willow Shrub Communities in the Julian Alps and on the Trnovski Gozd Plateau (NW and W Slovenia)
16/2 • 2017, 213–280 DOI: 10.1515/hacq-2017-0004 Phytosociological analysis of montane- subalpine dwarf willow shrub communities in the Julian Alps and on the Trnovski gozd plateau (NW and W Slovenia) Igor Dakskobler1 & Boštjan Surina1 Key words: phytosociology, Abstract synsystematics, Elyno-Seslerietea, By means of a phytosociological analysis of 72 relevés of montane-subalpine Rhododendro hirsuti-Ericetea carneae, shrub communities with dominating Rhododendron hirsutum, Salix waldsteiniana, Betulo carpaticae-Alnetea viridis, S. glabra and S. appendiculata from the Julian Alps and the the Trnovski Julian Alps, Dinaric Alps, Trnovski Gozd Plateau and by comparing them with similar communities elsewhere Gozd Plateau, Snežnik Mts., in the Alps and the Dinaric Alps we described a new association Laserpitio Slovenia. peucedanoidis-Salicetum waldsteinianae, a new subassociation Rhododendretum hirsuti vaccinietosum myrtilli, two new subassociations of the association Ključne besede: fitocenologija, Dryado-Rhodothamnetum chamaecisti that had recently been described in the sinsistematika, Elyno-Seslerietea, Dolomites (-caricetosum firmae, -salicetosum waldsteinianae), as well as a new Rhododendro hirsuti-Ericetea carneae, association Heliospermo pusillae-Rhododendretum hirsuti. We classified the glabrous Betulo carpaticae-Alnetea viridis, willow community in the study area into a new association Homogyno sylvestris- Julijske Alpe, Dinarsko gorstvo, Salicetum glabrae and proposed a new name – Rhododendro hirsuti-Salicetum Trnovski gozd, Snežniško -

Invasive Weeds of the Appalachian Region
$10 $10 PB1785 PB1785 Invasive Weeds Invasive Weeds of the of the Appalachian Appalachian Region Region i TABLE OF CONTENTS Acknowledgments……………………………………...i How to use this guide…………………………………ii IPM decision aid………………………………………..1 Invasive weeds Grasses …………………………………………..5 Broadleaves…………………………………….18 Vines………………………………………………35 Shrubs/trees……………………………………48 Parasitic plants………………………………..70 Herbicide chart………………………………………….72 Bibliography……………………………………………..73 Index………………………………………………………..76 AUTHORS Rebecca M. Koepke-Hill, Extension Assistant, The University of Tennessee Gregory R. Armel, Assistant Professor, Extension Specialist for Invasive Weeds, The University of Tennessee Robert J. Richardson, Assistant Professor and Extension Weed Specialist, North Caro- lina State University G. Neil Rhodes, Jr., Professor and Extension Weed Specialist, The University of Ten- nessee ACKNOWLEDGEMENTS The authors would like to thank all the individuals and organizations who have contributed their time, advice, financial support, and photos to the crea- tion of this guide. We would like to specifically thank the USDA, CSREES, and The Southern Region IPM Center for their extensive support of this pro- ject. COVER PHOTO CREDITS ii 1. Wavyleaf basketgrass - Geoffery Mason 2. Bamboo - Shawn Askew 3. Giant hogweed - Antonio DiTommaso 4. Japanese barberry - Leslie Merhoff 5. Mimosa - Becky Koepke-Hill 6. Periwinkle - Dan Tenaglia 7. Porcelainberry - Randy Prostak 8. Cogongrass - James Miller 9. Kudzu - Shawn Askew Photo credit note: Numbers in parenthesis following photo captions refer to the num- bered photographer list on the back cover. HOW TO USE THIS GUIDE Tabs: Blank tabs can be found at the top of each page. These can be custom- ized with pen or marker to best suit your method of organization. Examples: Infestation present On bordering land No concern Uncontrolled Treatment initiated Controlled Large infestation Medium infestation Small infestation Control Methods: Each mechanical control method is represented by an icon. -

Management of Invasive Plants and Pests of Illinois
MANAGEMENT OF INVASIVE PLANTS AND PESTS OF ILLINOIS AUTHORS Tricia Bethke, Forest Pest Outreach Coordinator, The Morton Arboretum Christopher Evans, Extension Forester, UIUC NRES ORIGINAL AUTHOR Karla Gage, Southern Illinois University 2 ACKNOWLEDGEMENTS This publication was funded, in part, through a grant from the Illinois Forestry Development Council (ifdc.nres.illinois.edu). Management of Invasive Plants and Pests of Illinois is an update and expansion of the original Management of Invasive Plants of Southern Illinois. The authors wish to acknowledge the Illinois Wildlife Preservation Fund, which supported the creation of the original document. The authors wish to thank The Morton Arboretum and Kurt Dreisilker, Mark Hochsprung, Mark McKinney and Clair Ryan for their edits and review of this document. The authors wish to thank The Morton Arboretum’s Natural Areas Conservation Training Program, which is generously funded by the Tellabs Foundation, for support, in part, of the publication of this guide. The authors wish to thank the USDA Animal Plant and Health Inspection Service which supported, in part, the update and publication of this document. The authors wish to thank the River to River Cooperative Weed Management Area and Kevin Rohling for assisting in the development of this publication. The Department of Natural Resources and Environmental Sciences and Extension Forestry at the University of Illinois would like to thank and acknowledge the Renewable Resources Extension Act (RREA) and the USDA National Institute of Food and Agriculture -

Globalna Strategija Ohranjanja Rastlinskih
GLOBALNA STRATEGIJA OHRANJANJA RASTLINSKIH VRST (TOČKA 8) UNIVERSITY BOTANIC GARDENS LJUBLJANA AND GSPC TARGET 8 HORTUS BOTANICUS UNIVERSITATIS LABACENSIS, SLOVENIA INDEX SEMINUM ANNO 2017 COLLECTORUM GLOBALNA STRATEGIJA OHRANJANJA RASTLINSKIH VRST (TOČKA 8) UNIVERSITY BOTANIC GARDENS LJUBLJANA AND GSPC TARGET 8 Recenzenti / Reviewers: Dr. sc. Sanja Kovačić, stručna savjetnica Botanički vrt Biološkog odsjeka Prirodoslovno-matematički fakultet, Sveučilište u Zagrebu muz. svet./ museum councilor/ dr. Nada Praprotnik Naslovnica / Front cover: Semeska banka / Seed bank Foto / Photo: J. Bavcon Foto / Photo: Jože Bavcon, Blanka Ravnjak Urednika / Editors: Jože Bavcon, Blanka Ravnjak Tehnični urednik / Tehnical editor: D. Bavcon Prevod / Translation: GRENS-TIM d.o.o. Elektronska izdaja / E-version Leto izdaje / Year of publication: 2018 Kraj izdaje / Place of publication: Ljubljana Izdal / Published by: Botanični vrt, Oddelek za biologijo, Biotehniška fakulteta UL Ižanska cesta 15, SI-1000 Ljubljana, Slovenija tel.: +386(0) 1 427-12-80, www.botanicni-vrt.si, [email protected] Zanj: znan. svet. dr. Jože Bavcon Botanični vrt je del mreže raziskovalnih infrastrukturnih centrov © Botanični vrt Univerze v Ljubljani / University Botanic Gardens Ljubljana ----------------------------------- Kataložni zapis o publikaciji (CIP) pripravili v Narodni in univerzitetni knjižnici v Ljubljani COBISS.SI-ID=297076224 ISBN 978-961-6822-51-0 (pdf) ----------------------------------- 1 Kazalo / Index Globalna strategija ohranjanja rastlinskih vrst (točka 8) -

Phenology Calendar (PDF)
Phenology Calendar Treatment for Common Invasive Plants in Northeast Illinois Name January February March April May June July August September October November December Mowing Do Not Mow Flowering Spray foliar herbicide Burn Alliaria petiolata (garlic mustard) Hand pull, dig Spray herbicide on cut stems Alnus glutinosa (black alder) Special Thanks to: Ed Hedborn - The Morton Arboretum Floyd Catchpole - Will County FPD Rob Littiken - TNC Kankakee Sands Becky Collings - The Field Museum Anthriscus sylvestris (wild chervil) Berberis thunbergii (Japanese barberry) Centaurea stoebe ssp. micranthos (syn. Centaurea maculosa /bieberstein /steube ) (spotted knapweed) Conium maculatum (poison hemlock) Dipsacus laciniatus (cut-leaf teasel) Dipsacus sylvestris /fullonum (common teasel) Eleagnus angustifolia (Russian olive) Elaeagnus umbellatus (Autumn olive) 1 Phenology Calendar Treatment for Common Invasive Plants in Northeast Illinois Name January February March April May June July August September October November December Mowing Do Not Mow Flowering Spray foliar herbicide Euonymus alatus (burning bush) Burn Hand pull, dig Spray herbicide on cut stems Euonymus fortunei (winter creeper) Euphorbia esula (leafy spurge) Frangula alnus (syn. Rhamnus frangula ) (glossy buckthorn) Hemerocallis spp. (daylily) Humulus japonicus (Japanese hops) Lespedeza sericea /cuneata (silky bush clover) Lonicera maackii (Amur honeysuckle) Lonicera tartarica -xylosteum complex (Tatarian honeysuckle) Lotus corniculatus (bird’s foot trefoil) Lysimachia nummularia (moneywort -

62/1 · 2021 Folia Biologica Et Geologica Ex: Razprave Razreda Za Naravoslovne Vede Dissertationes Classis IV (Historia Naturalis)
FOLIA BIOLOGICA ET GEOLOGICA = Ex RAZPRAVE IV. RAZREDA SAZU issn 1855-7996 · Letnik / Volume 62 · Številka / Number 1 · 2021 ISSN 1855-7996 | 20,00 € VSEBINA / CONTENTS Mitja Zupančič & Branko Vreš Ob stoletnici rojstva akademika Ernesta Mayerja RAZPRAVE / ESSAYS Igor Dakskobler & Valerija Babij Localities and sites of the neglected umbellifer Physospermum verticillatum (Apiaceae) on Mt. Slavnik in southwestern Slovenia Nahajališča in rastišča prezrte kobulnice Physospermum verticillatum na Slavniku v jugozahodni Sloveniji Igor Dakskobler & Andrej Martinčič New localities of Adiantum capillus-veneris and Moehringia villosa in the southern Julian Alps Nova nahajališča vrst Adiantum capillus-veneris in Moehringia villosa v južnih Julijskih Alpah Igor Dakskobler, Andrej Seliškar & Branko Vreš Phytosociological analysis of Gladiolus palustris sites in northwestern, western and southwestern Slovenia Fitocenološka oznaka rastišč vrste Gladiolus palustris v severozahodni, zahodni in jugozahodni Sloveniji Igor Dakskobler & Andrej Martinčič Botanične posebnosti Prodarjeve grape v zgornji Baški dolini (zahodna Slovenija) Botanical curiosities of Prodarjeva Grapa gorge in the upper Bača Valley (western Slovenia) OLIA BIOLOGICA ET GEOLOGICA 62/1 – 2021 BIOLOGICA OLIA Igor Dakskobler, Jože Čar, Anka Rudolf, Rafael Terpin & Branko Vreš F Rastje in rastlinstvo povodja Gačnika na Vojskem in v Trebuši – prispevek za njegovo naravovarstveno vrednotenje Vegetation and flora of the river-basin of the Gačnik stream (Vojsko, Spodnja Trebuša) – a contribution -

June 23, 2012
Maryland Native Plant Society Plant Lists We offer these lists to individuals and groups to enhance the enjoyment and study of plants of different locations in Maryland and nearby states. Their accuracy has not been verified by the Maryland Native Plant Society. SAVAGE RIVER STATE FOREST, GARRETT COUNTY, MD This list records plants seen during a field trip to the Savage River State Forest on June 23, 2012. Field trip led by Wade Dorsey and Liz McDowell. Plant list by Liz McDowell. Nomenclature follows the USDA Plant Database at http://plants.usda.gov (January 2012). Synonyms are footnoted for some species. Acer pensylvanicum Striped maple Aceraceae Acer rubrum Red maple Aceraceae Acer saccharum Sugar maple Aceraceae Achillea millefolium Yarrow Asteraceae Actaea racemosa1 Black cohosh Ranunculaceae Alliaria petiolata Garlic mustard Brassicaceae Amelanchier sp. Shadbush Rosaceae Apocynum cannabinum Indian hemp Apocynaceae Arabis canadensis Sicklepod Brassicaceae Athyrium filix-femina Lady fern Dryopteridaceae Barbarea vulgaris Common wintercress Brassicaceae Berberis thunbergii Japanese barberry Berberidaceae Betula lenta Black birch Betulaceae Carex platyphylla Broad-leaved sedge Cyperaceae Carya glabra Pignut hickory Juglandaceae Carya ovata Shagbark hickory Juglandaceae Castanea dentata American chestnut Fagaceae Chelidonium majus Celandine Papaveraceae Circaea lutetiana Enchanter’s nightshade Onagraceae Clinopodium vulgare2 Wild basil Lamiaceae Cypripedium acaule Pink lady’s-slipper Orchidaceae Dennstaedtia punctilobula Hay-scented -

Illinois Exotic Species List
Exotic Species in Illinois Descriptions for these exotic species in Illinois will be added to the Web page as time allows for their development. A name followed by an asterisk (*) indicates that a description for that species can currently be found on the Web site. This list does not currently name all of the exotic species in the state, but it does show many of them. It will be updated regularly with additional information. Microbes viral hemorrhagic septicemia Novirhabdovirus sp. West Nile virus Flavivirus sp. Zika virus Flavivirus sp. Fungi oak wilt Ceratocystis fagacearum chestnut blight Cryphonectria parasitica Dutch elm disease Ophiostoma novo-ulmi and Ophiostoma ulmi late blight Phytophthora infestans white-nose syndrome Pseudogymnoascus destructans butternut canker Sirococcus clavigignenti-juglandacearum Plants okra Abelmoschus esculentus velvet-leaf Abutilon theophrastii Amur maple* Acer ginnala Norway maple Acer platanoides sycamore maple Acer pseudoplatanus common yarrow* Achillea millefolium Japanese chaff flower Achyranthes japonica Russian knapweed Acroptilon repens climbing fumitory Adlumia fungosa jointed goat grass Aegilops cylindrica goutweed Aegopodium podagraria horse chestnut Aesculus hippocastanum fool’s parsley Aethusa cynapium crested wheat grass Agropyron cristatum wheat grass Agropyron desertorum corn cockle Agrostemma githago Rhode Island bent grass Agrostis capillaris tree-of-heaven* Ailanthus altissima slender hairgrass Aira caryophyllaea Geneva bugleweed Ajuga genevensis carpet bugleweed* Ajuga reptans mimosa -

Lake County Seed Collection Guide
/DNH&RXQW\,OOLQRLV86$ /DNH&RXQW\6HHG&ROOHFWLRQ*XLGH 1 6XPPHUPrairie)RUEV .HOO\6FKXOW]'DOH6KLHOGV /DNH&RXQW\)RUHVW3UHVHUYH'LVWULFW9ROXQWHHU6WHZDUGVKLS1HWZRUN Photos21'-6KLHOGV3URGXFHGE\'DOH6KLHOGV .HOO\6FKXOW]/DNH&RXQW\)RUHVW3UHVHUYH'LVWULFW>NVFKXOW]#OFISGRUJ@ .HOO\6FKXOW] 1 &&%<1&/LFHQVHGZRUNVDUHIUHHWRXVHVKDUHUHPL[ZLWKDWWULEXWLRQEXWGRHVQRWSHUPLWFRPPHUFLDOXVHRI WKHRULJLQDOZRUN >ILHOGJXLGHVILHOGPXVHXPRUJ@>5] version 2 3/2021 The pictures in this guide were assembled to help restoration volunteers identify ripe seeds of native species. The squares are 1” on a side in the indoor shots with white squares on the gray background. The seed shots are on a metric scale (mm divisions). Names used are those of Flora of the Chicago Region by Gerould Wilhelm and Laura Rericha. Our heartfelt thanks go to Laurie Ryan of the McHenry County Conservation District for her review. Harvest notes Successful collection of viable seed requires an understanding of when to collect, how to collect, how to store, how to process, and when to sow. Determine these criteria and have a plan before harvesting seeds, especially of uncommon species. The species are listed in order of the photo dates, so will give an approximate time for collection, but collection dates vary according to local weather effects on blooming and pollinators; proximity to Lake Michigan; slopes; sun vs shade, etc. Many seed harvest charts are available with collection dates, but it is best to scout each site rather than relying on historic dates. Seeds collected before mid-June should be sown right away. They are intolerant of dry storage and most of them require both warm & cold treatments to stimulate germination. Late June seeds are more tolerant of dry storage; sow these seeds soon, but you can let them dry for a few weeks. -
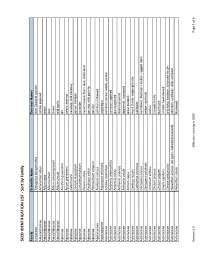
SEED IDENTIFICATION LIST - Sort by Family
SEED IDENTIFICATION LIST - Sort by Family Family Scientific Name Common Names Aizoaceae Tetragonia tetragonoides New Zealand spinach Amaranthaceae Amaranthus albus tumble pigweed Amaryllidaceae Allium cepa onion Amaryllidaceae Allium porrum leek Amaryllidaceae Allium schoenoprasum chives Amaryllidaceae Allium vineale wild garlic Apiaceae Anethum graveolens dill Apiaceae Apium graveolens celery, celeriac Apiaceae Carum carvi caraway; wild caraway Apiaceae Conium maculatum poison hemlock Apiaceae Coriandrum sativum coriander Apiaceae Daucus carota carrot; Queen Ane's lace; wild carrot Apiaceae Pastinaca sativa parsnip; wild parsnip Apiaceae Petroselinum crispum parsley Apocynaceae Asclepias syriaca common milkweed Asparagaceae Asparagus officinalis asparagus Asteraceae Achillea millefolium common yarrow, woolly yarrow Asteraceae Ambrosia artemisiifolia common ragweed Asteraceae Ambrosia trifida giant ragweed Asteraceae Anthemis arvensis field chamomile Asteraceae Anthemis cotula dogfennel, mayweed Asteraceae Arctium lappa great burdock Asteraceae Carduus nutans musk thistle, nodding thistle Asteraceae Carthamus tinctorius safflower Asteraceae Centaurea cyanus cornflower, bachelor's button, ragged robin Asteraceae Centaurea solstitialis yellow starthistle Asteraceae Cichorium endivia endive Asteraceae Cirsium arvense Canada thistle Asteraceae Cirsium vulgare bull thistle Asteraceae Crepis capillaris smooth hawksbeard Asteraceae Cynara cardunculus artichoke, cardoon, artichoke thistle Asteraceae Helianthus annuus (all types, cultivated and -

Long-Read Transcriptome and Other Genomic Resources for the Angiosperm Silene Noctiflora
bioRxiv preprint doi: https://doi.org/10.1101/2020.08.09.243378; this version posted August 10, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Long-read transcriptome and other genomic resources for the angiosperm Silene noctiflora Alissa M. Williams,*,1 Michael W. Itgen,* Amanda K. Broz,* Olivia G. Carter,* Daniel B. Sloan* *Department of Biology, Colorado State University, Fort Collins, Colorado 80523 1Corresponding author: [email protected] bioRxiv preprint doi: https://doi.org/10.1101/2020.08.09.243378; this version posted August 10, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 1 Abstract 2 3 The angiosperm genus Silene is a model system for several traits of ecological and evolutionary 4 significance in plants, including breeding system and sex chromosome evolution, host-pathogen 5 interactions, invasive species biology, heavy metal tolerance, and cytonuclear interactions. 6 Despite its importance, genomic resources for this large genus of approximately 850 species are 7 scarce, with only one published whole-genome sequence (from the dioecious species S. latifolia). 8 Here, we provide genomic and transcriptomic resources for a hermaphroditic representative of 9 this genus (S. noctiflora), including a PacBio Iso-Seq transcriptome, which uses long-read, 10 single-molecule sequencing technology to analyze full-length mRNA transcripts and identify 11 paralogous genes and alternatively spliced genes. -

The Experience Elicited by Hallucinogens Presents the Highest Similarity to Dreaming Within a Large Database of Psychoactive Substance Reports
ORIGINAL RESEARCH published: 22 January 2018 doi: 10.3389/fnins.2018.00007 The Experience Elicited by Hallucinogens Presents the Highest Similarity to Dreaming within a Large Database of Psychoactive Substance Reports Camila Sanz 1, Federico Zamberlan 1, Earth Erowid 2, Fire Erowid 2 and Enzo Tagliazucchi 1,3* 1 Departamento de Física, Universidad de Buenos Aires, Buenos Aires, Argentina, 2 Erowid Center, Grass Valley, CA, United States, 3 Brain and Spine Institute, Paris, France Ever since the modern rediscovery of psychedelic substances by Western society, Edited by: several authors have independently proposed that their effects bear a high resemblance Rick Strassman, to the dreams and dreamlike experiences occurring naturally during the sleep-wake University of New Mexico School of cycle. Recent studies in humans have provided neurophysiological evidence supporting Medicine, United States this hypothesis. However, a rigorous comparative analysis of the phenomenology (“what Reviewed by: Matthias E. Liechti, it feels like” to experience these states) is currently lacking. We investigated the semantic University Hospital Basel, Switzerland similarity between a large number of subjective reports of psychoactive substances and Michael Kometer, University of Zurich, Switzerland reports of high/low lucidity dreams, and found that the highest-ranking substance in *Correspondence: terms of the similarity to high lucidity dreams was the serotonergic psychedelic lysergic Enzo Tagliazucchi acid diethylamide (LSD), whereas the highest-ranking in terms of the similarity to dreams [email protected] of low lucidity were plants of the Datura genus, rich in deliriant tropane alkaloids. Specialty section: Conversely, sedatives, stimulants, antipsychotics, and antidepressants comprised most This article was submitted to of the lowest-ranking substances.