Cellular and Molecular Mechanisms of Tooth Root Development Jingyuan
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
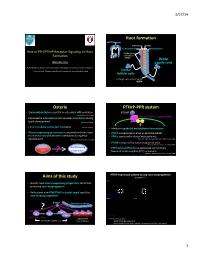
Ono -- PTH-Pthrp Receptor Signaling in Osterix-Expressing Progenitors.Pdf
3/27/14 Root forma)on Cementum Dentin Cementoblast Odontoblast Role of PTH/PTHrP Receptor Signaling on Root Epithelial rests Formaon of Malassez (ERM) Dental Wanida Ono papilla cells AGE Orthodon;cs, Department of Developmental Biology, Harvard School of Dental Medicine Endocrine Unit, MassachuseLs General Hospital and Harvard Medical School Dental follicle cells Hertwig’s epithelial root sheath (HERS) Osterix PTHrP-PPR system • Transcripon factor essen;al to osteoblast differen;aon PTHrP (Nakashima K et al. 2002) PTH/PTHrP receptor (PPR) • Expressed in odontoblasts and alveolar osteoblasts during Gαs Gq tooth development (Chen S et al. 2009) • Controls cellular cementum formaon (Cao Z et al. 2012) • Mediates epithelial-mesenchymal interacons • Osterix-expressing precursors in the perichondrium move • PTHrP is expressed in enamel epithelia/HERS? to bone marrow and become osteoblasts during fetal • PPR is expressed in dental mesenchymes development (Maes C, Kronenberg HM et al. 2010) (Beck et al 1995; Lee Deeds and Segre 1995; Liu et al 1998) • PTHrP is required for tooth erup;on in mice (Philbrick WM, Karaplis AC et al. PNAS 1998) Osterix+ Root-forming • PPR haploinsufficiency is associated with primary cells progenitors failure of tooth erup;on (PFE) in humans ? (Decker E, Weber BH et al. Am J Hum Gen 2008) PTHrP expression paern during root morphogenesis Aims of this study PTHrPLacZ/+ x40 P7 P14 P49 • Iden;fy how osterix-expressing progenitors contribute to murine root morphogenesis • Understand how PTH/PTHrP receptor signal regulates root-forming progenitors PTHrP-LacZ x200 P7 x400 P14 ? PTHrP Epithelial root sheath PPR PTHrP-LacZ Osx+ progenitors Odontoblast PTHrP par;cipates in ……. -

Experimental Induction of Odontoblast Differentiation and Stimulation During Preparative Processes
Cells and Materials Volume 3 Number 2 Article 8 1993 Experimental Induction of Odontoblast Differentiation and Stimulation During Preparative Processes H. Lesot Institut de Biologie Médicale C. Begue-Kirn Institut de Biologie Médicale M. D. Kubler Institut de Biologie Médicale J. M. Meyer Institut de Biologie Médicale A. J. Smith Dental School, Birmingham See next page for additional authors Follow this and additional works at: https://digitalcommons.usu.edu/cellsandmaterials Part of the Biomedical Engineering and Bioengineering Commons Recommended Citation Lesot, H.; Begue-Kirn, C.; Kubler, M. D.; Meyer, J. M.; Smith, A. J.; Cassidy, N.; and Ruch, J. V. (1993) "Experimental Induction of Odontoblast Differentiation and Stimulation During Preparative Processes," Cells and Materials: Vol. 3 : No. 2 , Article 8. Available at: https://digitalcommons.usu.edu/cellsandmaterials/vol3/iss2/8 This Article is brought to you for free and open access by the Western Dairy Center at DigitalCommons@USU. It has been accepted for inclusion in Cells and Materials by an authorized administrator of DigitalCommons@USU. For more information, please contact [email protected]. Experimental Induction of Odontoblast Differentiation and Stimulation During Preparative Processes Authors H. Lesot, C. Begue-Kirn, M. D. Kubler, J. M. Meyer, A. J. Smith, N. Cassidy, and J. V. Ruch This article is available in Cells and Materials: https://digitalcommons.usu.edu/cellsandmaterials/vol3/iss2/8 Cells and Materials, Vol. 3, No. 2, 1993 (Pages201-217) 1051-6794/93$5. 00 +. 00 Scanning Microscopy International, Chicago (AMF O'Hare), IL 60666 USA EXPERIMENTAL INDUCTION OF ODONTOBLAST DIFFERENTIATION AND STIMULATION DURING REPARATIVE PROCESSES 1 1 1 2 2 1 H. -

Journal of Dental Research
Journal of Dental Research http://jdr.sagepub.com/ Cell Differentiation and Matrix Organization in Engineered Teeth A. Nait Lechguer, M.L. Couble, N. Labert, S. Kuchler-Bopp, L. Keller, H. Magloire, F. Bleicher and H. Lesot J DENT RES 2011 90: 583 originally published online 4 February 2011 DOI: 10.1177/0022034510391796 The online version of this article can be found at: http://jdr.sagepub.com/content/90/5/583 Published by: http://www.sagepublications.com On behalf of: International and American Associations for Dental Research Additional services and information for Journal of Dental Research can be found at: Email Alerts: http://jdr.sagepub.com/cgi/alerts Subscriptions: http://jdr.sagepub.com/subscriptions Reprints: http://www.sagepub.com/journalsReprints.nav Permissions: http://www.sagepub.com/journalsPermissions.nav >> Version of Record - Apr 13, 2011 OnlineFirst Version of Record - Feb 4, 2011 What is This? Downloaded from jdr.sagepub.com at Service Commun de la Documentation Université de Strasbourg on September 6, 2013 For personal use only. No other uses without permission. © 2011 International & American Associations for Dental Research RESEARCH REPORTS Biomaterials & Bioengineering A. Nait Lechguer1,2, M.L. Couble3,4, N. Labert3,4, S. Kuchler-Bopp1,2, Cell Differentiation and L. Keller1,2, H. Magloire3,4, F. Bleicher3,4, Matrix Organization in and H. Lesot1,2* Engineered Teeth 1INSERM UMR 977, Faculté de Médecine, 11, rue Humann, F-67085 Strasbourg, France; 2Dental School, University of Strasbourg, Strasbourg, France; 3Université de Lyon, Faculté d’Odontologie, Rue Guillaume Paradin, F-69372 Lyon Cedex 08, France; and 4IGFL, CNRS UMR 5242, Ecole Normale Supérieure, 46 Allée d’Italie, 69364, Lyon Cedex 08, France; *corresponding author, [email protected] J Dent Res 90(5):583-589, 2011 ABSTRACT InTRODuCTIOn Embryonic dental cells were used to check a series of criteria to be achieved for tooth engineering. -

Benign Cementoblastoma Associated with an Unerupted Third Molar - a Case Report
CORE Metadata, citation and similar papers at core.ac.uk Provided by Directory of Open Access Journals BENIGN CEMENTOBLASTOMA ASSOCIATED WITH AN UNERUPTED THIRD MOLAR - A CASE REPORT J.Dinakar* M.S.Senthil Kumar** Shiju Mathew Jacob*** *Professor & HOD, Department of Oral Pathology, ** Reader, *** Lecturer, Department of Oral Surgery, Sri Ramakrishna Dental College and Hospital, Coimbatore, Tamilnadu, India. ABSTRACT: Cementoblastoma is a rare odontogenic tumor derived from odontogenic ectomesenchyme of cementoblast origin that forms cementum layer on the roots of a tooth. A case report is presented of a patient treated with surgical excision of Cementoblastoma associated with an unerupted infected right lower third molar tooth. Key words: Cementoblastoma, Odontogenic tumour, unerupted third molar. The cell of origin is cementoblast. INTRODUCTION: Clinically it causes bony expansion. The commonest site is the posterior region of Cementoblastoma is an odontogenic the mandible. In the radiograph it is seen tumor of ectomesenchymal origin. It is as large radiopaque mass associated with also called cementoma. They are large the root of the tooth. We report a case of bulbous mass of cementum or cementum- Benign Cementoblastoma from Sri like tissue on roots of teeth. Ramakrishna Dental College & Hospital, Coimbatore. restriction in opening the mouth and intra oral examination reveals a partially CASE REPORT: erupted third molar tooth with pus discharge. A panoramic radiograph A 41 year old man presented to our showed a radio-opaque, dense, department with a complaint of pain and amorphous, irregularly shaped mass swelling in the right lower half of the face. measuring 2.2 x 1.5cm attached with the Patient gave history of intermittent pain third molar (Fig 1,1a). -

Intrusion of Incisors to Facilitate Restoration: the Impact on the Periodontium
Note: This is a sample Eoster. Your EPoster does not need to use the same format style. For example your title slide does not need to have the title of your EPoster in a box surrounded with a pink border. Intrusion of Incisors to Facilitate Restoration: The Impact on the Periodontium Names of Investigators Date Background and Purpose This 60 year old male had severe attrition of his maxillary and mandibular incisors due to a protrusive bruxing habit. The patient’s restorative dentist could not restore the mandibular incisors without significant crown lengthening. However, with orthodontic intrusion of the incisors, the restorative dentist was able to restore these teeth without further incisal edge reduction, crown lengthening, or endodontic treatment. When teeth are intruded in adults, what is the impact on the periodontium? The purpose of this study was to determine the effect of adult incisor intrusion on the alveolar bone level and on root length. Materials and Methods We collected the orthodontic records of 43 consecutively treated adult patients (aged > 19 years) from four orthodontic practices. This project was approved by the IRB at our university. Records were selected based upon the following criteria: • incisor intrusion attempted to create interocclusal space for restorative treatment or correction of excessive anterior overbite • pre- and posttreatment periapical and cephalometric radiographs were available • no incisor extraction or restorative procedures affecting the cementoenamel junction during the treatment period pretreatment pretreatment Materials and Methods We used cephalometric and periapical radiographs to measure incisor intrusion. The radiographs were imported and the digital images were analyzed with Image J, a public-domain Java image processing program developed at the US National Institutes of Health. -

Tooth Enamel and Its Dynamic Protein Matrix
International Journal of Molecular Sciences Review Tooth Enamel and Its Dynamic Protein Matrix Ana Gil-Bona 1,2,* and Felicitas B. Bidlack 1,2,* 1 The Forsyth Institute, Cambridge, MA 02142, USA 2 Department of Developmental Biology, Harvard School of Dental Medicine, Boston, MA 02115, USA * Correspondence: [email protected] (A.G.-B.); [email protected] (F.B.B.) Received: 26 May 2020; Accepted: 20 June 2020; Published: 23 June 2020 Abstract: Tooth enamel is the outer covering of tooth crowns, the hardest material in the mammalian body, yet fracture resistant. The extremely high content of 95 wt% calcium phosphate in healthy adult teeth is achieved through mineralization of a proteinaceous matrix that changes in abundance and composition. Enamel-specific proteins and proteases are known to be critical for proper enamel formation. Recent proteomics analyses revealed many other proteins with their roles in enamel formation yet to be unraveled. Although the exact protein composition of healthy tooth enamel is still unknown, it is apparent that compromised enamel deviates in amount and composition of its organic material. Why these differences affect both the mineralization process before tooth eruption and the properties of erupted teeth will become apparent as proteomics protocols are adjusted to the variability between species, tooth size, sample size and ephemeral organic content of forming teeth. This review summarizes the current knowledge and published proteomics data of healthy and diseased tooth enamel, including advancements in forensic applications and disease models in animals. A summary and discussion of the status quo highlights how recent proteomics findings advance our understating of the complexity and temporal changes of extracellular matrix composition during tooth enamel formation. -

6 Development of the Teeth: Root and Supporting Structures Nagat M
AVERY Chap.06 27-11-2002 10:09 Pagina 108 108 II Development of the Teeth and Supporting Structures 6 Development of the Teeth: Root and Supporting Structures Nagat M. ElNesr and James K. Avery Chapter Outline Introduction Introduction... 108 Objectives... 108 Root development is initiated through the contributions Root Sheath Development... 109 of the cells originating from the enamel organ, dental Single-Root Formation... 110 papilla, and dental follicle. The cells of the outer enamel Multiple-Root Formation... 111 epithelium contact the inner enamel epithelium at the Root Formation Anomalies... 112 base of the enamel organ, the cervical loop (Figs. 6.1 and Fate of the Epithelial Root Sheath (Hertwig's Sheath)... 113 6.2A). Later, with crown completion, the cells of the cer- Dental Follicle... 114 vical loop continue to grow away from the crown and Development of (Intermediate) Cementum... 116 become root sheath cells (Figs. 6.2B and 6.3). The inner Cellular and Acellular Cementum... 116 root sheath cells cause root formation by inducing the Development of the Periodontal Ligament... 117 adjacent cells of the dental papilla to become odonto- Development of the Alveolar Process... 119 blasts, which in turn will form root dentin. The root Summary... 121 sheath will further dictate whether the tooth will have Self-Evaluation Review... 122 single or multiple roots. The remainder of the cells of the dental papilla will then become the cells of the root pulp.The third compo- nent in root formation, the dental follicle, is the tissue that surrounds the enamel organ, the dental papilla, and the root. -

Lecture 2 – Bone
Oral Histology Summary Notes Enoch Ng Lecture 2 – Bone - Protection of brain, lungs, other internal organs - Structural support for heart, lungs, and marrow - Attachment sites for muscles - Mineral reservoir for calcium (99% of body’s) and phosphorous (85% of body’s) - Trap for dangerous minerals (ex:// lead) - Transduction of sound - Endocrine organ (osteocalcin regulates insulin signaling, glucose metabolism, and fat mass) Structure - Compact/Cortical o Diaphysis of long bone, “envelope” of cuboid bones (vertebrae) o 10% porosity, 70-80% calcified (4x mass of trabecular bone) o Protective, subject to bending/torsion/compressive forces o Has Haversian system structure - Trabecular/Cancellous o Metaphysis and epiphysis of long bone, cuboid bone o 3D branching lattice formed along areas of mechanical stress o 50-90% porosity, 15-25% calcified (1/4 mass of compact bone) o High surface area high cellular activity (has marrow) o Metabolic turnover 8x greater than cortical bone o Subject to compressive forces o Trabeculae lined with endosteum (contains osteoprogenitors, osteoblasts, osteoclasts) - Woven Bone o Immature/primitive, rapidly growing . Normally – embryos, newborns, fracture calluses, metaphyseal region of bone . Abnormally – tumors, osteogenesis imperfecta, Pagetic bone o Disorganized, no uniform orientation of collagen fibers, coarse fibers, cells randomly arranged, varying mineral content, isotropic mechanical behavior (behavior the same no matter direction of applied force) - Lamellar Bone o Mature bone, remodeling of woven -

Specialized Stem Cell Niche Enables Repetitive Renewal of Alligator Teeth
Specialized stem cell niche enables repetitive renewal PNAS PLUS of alligator teeth Ping Wua, Xiaoshan Wua,b, Ting-Xin Jianga, Ruth M. Elseyc, Bradley L. Templed, Stephen J. Diverse, Travis C. Glennd, Kuo Yuanf, Min-Huey Cheng,h, Randall B. Widelitza, and Cheng-Ming Chuonga,h,i,1 aDepartment of Pathology, University of Southern California, Los Angeles, CA 90033; bDepartment of Oral and Maxillofacial Surgery, Xiangya Hospital, Central South University, Hunan 410008, China; cLouisiana Department of Wildlife and Fisheries, Rockefeller Wildlife Refuge, Grand Chenier, LA 70643; dEnvironmental Health Science and eDepartment of Small Animal Medicine and Surgery, University of Georgia, Athens, GA 30602; fDepartment of Dentistry and iResearch Center for Wound Repair and Regeneration, National Cheng Kung University, Tainan City 70101, Taiwan; and gSchool of Dentistry and hResearch Center for Developmental Biology and Regenerative Medicine, National Taiwan University, Taipei 10617, Taiwan Edited by Edward M. De Robertis, Howard Hughes Medical Institute/University of California, Los Angeles, CA, and accepted by the Editorial Board March 28, 2013 (received for review July 31, 2012) Reptiles and fish have robust regenerative powers for tooth renewal. replaced from the dental lamina connected to the lingual side of However, extant mammals can either renew their teeth one time the deciduous tooth (15). Human teeth are only replaced one time; (diphyodont dentition) or not at all (monophyodont dentition). however, a remnant of the dental lamina still exists (16) and may Humans replace their milk teeth with permanent teeth and then become activated later in life to form odontogenic tumors (17). lose their ability for tooth renewal. -

Structural Changes in the Oral Microbiome of the Adolescent
www.nature.com/scientificreports OPEN Structural changes in the oral microbiome of the adolescent patients with moderate or severe dental fuorosis Qian Wang1,2, Xuelan Chen1,4, Huan Hu2, Xiaoyuan Wei3, Xiaofan Wang3, Zehui Peng4, Rui Ma4, Qian Zhao4, Jiangchao Zhao3*, Jianguo Liu1* & Feilong Deng1,2,3* Dental fuorosis is a very prevalent endemic disease. Although oral microbiome has been reported to correlate with diferent oral diseases, there appears to be an absence of research recognizing any relationship between the severity of dental fuorosis and the oral microbiome. To this end, we investigated the changes in oral microbial community structure and identifed bacterial species associated with moderate and severe dental fuorosis. Salivary samples of 42 individuals, assigned into Healthy (N = 9), Mild (N = 14) and Moderate/Severe (M&S, N = 19), were investigated using the V4 region of 16S rRNA gene. The oral microbial community structure based on Bray Curtis and Weighted Unifrac were signifcantly changed in the M&S group compared with both of Healthy and Mild. As the predominant phyla, Firmicutes and Bacteroidetes showed variation in the relative abundance among groups. The Firmicutes/Bacteroidetes (F/B) ratio was signifcantly higher in the M&S group. LEfSe analysis was used to identify diferentially represented taxa at the species level. Several genera such as Streptococcus mitis, Gemella parahaemolysans, Lactococcus lactis, and Fusobacterium nucleatum, were signifcantly more abundant in patients with moderate/severe dental fuorosis, while Prevotella melaninogenica and Schaalia odontolytica were enriched in the Healthy group. In conclusion, our study indicates oral microbiome shift in patients with moderate/severe dental fuorosis. -

External Root Resorption of Young Premolar Teeth in Dentition With
10.5005/jp-journals-10015-1236 KapilaORIGINAL Arambawatta RESEARCH et al External Root Resorption of Young Premolar Teeth in Dentition with Crowding Kapila Arambawatta, Roshan Peiris, Dhammika Ihalagedara, Anushka Abeysundara, Deepthi Nanayakkara ABSTRACT least understood type of root resorption, characterized by its The present study was conducted to investigate the prevalence FHUYLFDOORFDWLRQDQGLQYDVLYHQDWXUH7KLVUHVRUSWLYHSURFHVV of external cervical resorption (ECR) in different tooth surfaces which leads to prRJUHVVLYHDQGXVXDOO\GHVWUXFWLYHORVVRI RIPD[LOODU\¿UVWSUHPRODUVLQD6UL/DQNDQSRSXODWLRQ tooth structure has been a source of interest to clinicians A sample of 59 (15 males, 44 females) permanent maxillary 2-5 ¿UVWSUHPRODUV DJHUDQJH\HDUV ZHUHXVHG7KHWHHWK DQGUHVHDUFKHUVIRURYHUDFHQWXU\ 7KHH[DFWFDXVHRI had been extracted for orthodontic reasons and were stored (&5LVSRRUO\XQGHUVWRRG$OWKRXJKWKHHWLRORJ\DQG LQIRUPDOLQ0RUSKRORJLFDOO\VRXQGWHHWKZHUHVHOHFWHG SDWKRJHQHVLVUHPDLQREVFXUHVHYHUDOSRWHQWLDOSUHGLVSRVLQJ IRUWKHVWXG\7KHWHHWKZHUHVWDLQHGZLWKFDUEROIXFKVLQ7KH IDFWRUVKDYHEHHQSXWIRUZDUGDQGRIWKHVHWKHLQWUDFRURQDO cervical regions of the stained teeth were observed under 10× 6 PDJQL¿FDWLRQV XVLQJ D GLVVHFWLQJ PLFURVFRSH 2O\PSXV 6= bleaching has been the most widely documented factor. In WRLGHQWLI\DQ\UHVRUSWLRQDUHDV7KHUHVRUSWLRQDUHDVSUHVHQW addition, dental trauma, orthodontic treatment, periodontal on buccal, lingual, mesial and distal aspects of all teeth were WUHDWPHQWVXUJHU\LQYROYLQJWKHFHPHQWRHQDPHOMXQFWLRQ UHFRUGHG DQGLGLRSDWKLFHWLRORJ\KDYHDOVREHHQGHVFULEHG2,7-11 -

The Cementoenamel Junction: Gap, Overlay and Edge to Edge Relationships
University of Tennessee, Knoxville TRACE: Tennessee Research and Creative Exchange Masters Theses Graduate School 8-1996 The Cementoenamel Junction: Gap, Overlay and Edge to Edge Relationships Elizabeth A. Gilb University of Tennessee, Knoxville Follow this and additional works at: https://trace.tennessee.edu/utk_gradthes Part of the Anthropology Commons Recommended Citation Gilb, Elizabeth A., "The Cementoenamel Junction: Gap, Overlay and Edge to Edge Relationships. " Master's Thesis, University of Tennessee, 1996. https://trace.tennessee.edu/utk_gradthes/4128 This Thesis is brought to you for free and open access by the Graduate School at TRACE: Tennessee Research and Creative Exchange. It has been accepted for inclusion in Masters Theses by an authorized administrator of TRACE: Tennessee Research and Creative Exchange. For more information, please contact [email protected]. To the Graduate Council: I am submitting herewith a thesis written by Elizabeth A. Gilb entitled "The Cementoenamel Junction: Gap, Overlay and Edge to Edge Relationships." I have examined the final electronic copy of this thesis for form and content and recommend that it be accepted in partial fulfillment of the requirements for the degree of Master of Arts, with a major in Anthropology. Murray Marks, Major Professor We have read this thesis and recommend its acceptance: Richard Jantz, William M. Bass, David Gerard Accepted for the Council: Carolyn R. Hodges Vice Provost and Dean of the Graduate School (Original signatures are on file with official studentecor r ds.) To the Graduate Council: I am submitting herewith a thesis written by Elizabeth A. Gilb entitled "The Cementoenamel Junction: Gap, Overlay and Edge to Edge Relationships." I have examined the finalcopy of this thesis forform and content and recommend that it be accepted in partial fulfillmentof the requirements forthe degree of Master of Arts, with a major in Anthropology.