Detection and Discrimination of Sulfate Minerals Using Reflectance
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A La Torre Aaker Aalbers Aaldert Aarmour Aaron
A LA TORRE ABDIE ABLEMAN ABRAMOWITCH AAKER ABE ABLES ABRAMOWITZ AALBERS ABEE ABLETSON ABRAMOWSKY AALDERT ABEEL ABLETT ABRAMS AARMOUR ABEELS ABLEY ABRAMSEN AARON ABEKE ABLI ABRAMSKI AARONS ABEKEN ABLITT ABRAMSON AARONSON ABEKING ABLOTT ABRAMZON AASEN ABEL ABNER ABRASHKIN ABAD ABELA ABNETT ABRELL ABADAM ABELE ABNEY ABREU ABADIE ABELER ABORDEAN ABREY ABALOS ABELES ABORDENE ABRIANI ABARCA ABELI ABOT ABRIL ABATE ABELIN ABOTS ABRLI ABB ABELL ABOTSON ABRUZZO ABBA ABELLA ABOTT ABSALOM ABBARCROMBIE ABELLE ABOTTS ABSALON ABBAS ABELLS ABOTTSON ABSHALON ABBAT ABELMAN ABRAHAM ABSHER ABBATE ABELS ABRAHAMER ABSHIRE ABBATIELLO ABELSON ABRAHAMI ABSOLEM ABBATT ABEMA ABRAHAMIAN ABSOLOM ABBAY ABEN ABRAHAMOF ABSOLON ABBAYE ABENDROTH ABRAHAMOFF ABSON ABBAYS ABER ABRAHAMOV ABSTON ABBDIE ABERCROMBIE ABRAHAMOVITZ ABT ABBE ABERCROMBY ABRAHAMOWICZ ABTS ABBEKE ABERCRUMBIE ABRAHAMS ABURN ABBEL ABERCRUMBY ABRAHAMS ABY ABBELD ABERCRUMMY ABRAHAMSEN ABYRCRUMBIE ABBELL ABERDEAN ABRAHAMSOHN ABYRCRUMBY ABBELLS ABERDEEN ABRAHAMSON AC ABBELS ABERDEIN ABRAHAMSSON ACASTER ABBEMA ABERDENE ABRAHAMY ACCA ABBEN ABERG ABRAHM ACCARDI ABBERCROMBIE ABERLE ABRAHMOV ACCARDO ABBERCROMMIE ABERLI ABRAHMOVICI ACE ABBERCRUMBIE ABERLIN ABRAHMS ACERO ABBERDENE ABERNATHY ABRAHMSON ACESTER ABBERDINE ABERNETHY ABRAM ACETO ABBERLEY ABERT ABRAMCHIK ACEVEDO ABBETT ABEYTA ABRAMCIK ACEVES ABBEY ABHERCROMBIE ABRAMI ACHARD ABBIE ABHIRCROMBIE ABRAMIN ACHENBACH ABBING ABIRCOMBIE ABRAMINO ACHENSON ABBIRCROMBIE ABIRCROMBIE ABRAMO ACHERSON ABBIRCROMBY ABIRCROMBY ABRAMOF ACHESON ABBIRCRUMMY ABIRCROMMBIE ABRAMOFF -

IUGG03-Program.Pdf
The Science Council of Japan and sixteen Japanese scientific societies will host IUGG2003, the XXIII General Assembly of the International Union of Geodesy and Geophysics. Hosts Science Council of Japan The Geodetic Society of Japan Seismological Society of Japan The Volcanological Society of Japan Meteorological Society of Japan Society of Geomagnetism and Earth, Planetary and Space Sciences Japan Society of Hydrology and Water Resources The Japanese Association of Hydrological Sciences The Japanese Society of Snow and Ice The Oceanographic Society of Japan The Japanese Society for Planetary Sciences The Japanese Society of Limnology Japan Society of Civil Engineers Japanese Association of Groundwater Hydrology The Balneological Society of Japan Japan Society of Erosion Control Engineering The Geochemical Society of Japan Special Support Hokkaido Prefecture City of Sapporo Co-Sponsor National Research Institute for Earth Science and Disaster Prevention (JSS01 Hagiwara Symposium on Monitoring and Modeling of Earthquake and Volcanic Processes for Prediction) Center for Climate System Research, University of Tokyo (JSM01 Toward High Resolution Climate Models and Earth System Models) Support Ministry of Education, Culture, Sports, Science and Technology Ministry of Economy, Trade and Industry Ministry of Land, Infrastructure and Transport Japan Marine Science and Technology Center National Institute of Advanced Industrial Science and Technology Japan Earth and Planetary Science Joint Meeting Organization Japanese Forestry Society Japan Business -

Fichier National Des Sites Classés
Ministère de la transition écologique et solidaire FICHIER NATIONAL DES SITES CLASSES ( mise à jour 29 décembre 2019) superficie (ha) A : arrêté Région dep commune nom DNP critère date territoire dpm total D : décret La tourbière de Malebronde située à Brenod, parcelle 9 Auvergne-Rhône- 1 Brenod TC A 30 avril 1934 0,2 0,2 Alpes section B Auvergne-Rhône- Alpes 1 Chaley La cascade de Charabotte, à Chaley A A 14 juin 1909 0,24 0,24 Auvergne-Rhône- Alpes 1 Charix Cascade du Moulin de Charix A A 14 juin 1909 0 0 L'ensemble formé par le lac Genin et ses abords situés Auvergne-Rhône- Alpes 1 Charix, Échallon, Oyonnax sur le territoire des communes de Charix, Échallon et TC A 1 mars 1935 50 50 Oyonnax Extension du site du Défilé de Fort l'Écluse sur les communes de Collonges et de Léaz (ne remplaçant pas Auvergne-Rhône- 1 Collonges, Léaz P D 19 mai 1992 1844 1844 Alpes l'arrêté du 6 novembre 1946 que pour la partie concernant la commune de Chevrier en Haute-Savoie) Auvergne-Rhône- Alpes 1 Corveissiat La grotte de Corveissiat A A 8 juin 1909 0 0 Auvergne-Rhône- Alpes 1 Courmangoux, Pressiat Le Mont Myon et ses abords à Courmangoux et Pressiat P A 10 avril 1946 46 46 La Pierre des Marais sur la commune de Divonne-les- Auvergne-Rhône- 1 Divonne-les-Bains A A 14 juin 1909 Alpes Bains L'ensemble formé sur la commune de Fareins par le site Auvergne-Rhône- 1 Fareins P D 1 mars 1982 78,49 78,49 Alpes des abords du château de Fléchères Fareins, Genouilleux, Guéreins, Lurcy, Messimy-sur-Saône, L'ensemble dit "Val de Saône" parmi les sites des Auvergne-Rhône- -

Elevation and Deformation Extraction from Tomosar
Title Elevation and Deformation Extraction from TomoSAR Lang Feng Thesis submitted for the degree of Doctor of Philosophy Mullard Space Science Laboratory Department of Space and Climate Physics University College London 2019 1 This page is intentionally left blank. 2 Author Declaration I, Lang Feng, confirm that the work presented in this thesis is my own. Where information has been derived from other sources, I confirm that this has been indicated in the thesis. LANG FENG 2019-02-01 3 This page is intentionally left blank. 4 Abstract 3D SAR tomography (TomoSAR) and 4D SAR differential tomography (Diff- TomoSAR) exploit multi-baseline SAR data stacks to provide an essential innovation of SAR Interferometry for many applications, sensing complex scenes with multiple scatterers mapped into the same SAR pixel cell. However, these are still influenced by DEM uncertainty, temporal decorrelation, orbital, tropospheric and ionospheric phase distortion and height blurring. In this thesis, these techniques are explored. As part of this exploration, the systematic procedures for DEM generation, DEM quality assessment, DEM quality improvement and DEM applications are first studied. Besides, this thesis focuses on the whole cycle of systematic methods for 3D & 4D TomoSAR imaging for height and deformation retrieval, from the problem formation phase, through the development of methods to testing on real SAR data. After DEM generation introduction from spaceborne bistatic InSAR (TanDEM- X) and airborne photogrammetry (Bluesky), a new DEM co-registration method with line feature validation (river network line, ridgeline, valley line, crater boundary feature and so on) is developed and demonstrated to assist the study of a wide area DEM data quality. -

Adams Adkinson Aeschlimann Aisslinger Akkermann
BUSCAPRONTA www.buscapronta.com ARQUIVO 27 DE PESQUISAS GENEALÓGICAS 189 PÁGINAS – MÉDIA DE 60.800 SOBRENOMES/OCORRÊNCIA Para pesquisar, utilize a ferramenta EDITAR/LOCALIZAR do WORD. A cada vez que você clicar ENTER e aparecer o sobrenome pesquisado GRIFADO (FUNDO PRETO) corresponderá um endereço Internet correspondente que foi pesquisado por nossa equipe. Ao solicitar seus endereços de acesso Internet, informe o SOBRENOME PESQUISADO, o número do ARQUIVO BUSCAPRONTA DIV ou BUSCAPRONTA GEN correspondente e o número de vezes em que encontrou o SOBRENOME PESQUISADO. Número eventualmente existente à direita do sobrenome (e na mesma linha) indica número de pessoas com aquele sobrenome cujas informações genealógicas são apresentadas. O valor de cada endereço Internet solicitado está em nosso site www.buscapronta.com . Para dados especificamente de registros gerais pesquise nos arquivos BUSCAPRONTA DIV. ATENÇÃO: Quando pesquisar em nossos arquivos, ao digitar o sobrenome procurado, faça- o, sempre que julgar necessário, COM E SEM os acentos agudo, grave, circunflexo, crase, til e trema. Sobrenomes com (ç) cedilha, digite também somente com (c) ou com dois esses (ss). Sobrenomes com dois esses (ss), digite com somente um esse (s) e com (ç). (ZZ) digite, também (Z) e vice-versa. (LL) digite, também (L) e vice-versa. Van Wolfgang – pesquise Wolfgang (faça o mesmo com outros complementos: Van der, De la etc) Sobrenomes compostos ( Mendes Caldeira) pesquise separadamente: MENDES e depois CALDEIRA. Tendo dificuldade com caracter Ø HAMMERSHØY – pesquise HAMMERSH HØJBJERG – pesquise JBJERG BUSCAPRONTA não reproduz dados genealógicos das pessoas, sendo necessário acessar os documentos Internet correspondentes para obter tais dados e informações. DESEJAMOS PLENO SUCESSO EM SUA PESQUISA. -

2016 KWPN-NA Top Fives
2016 KWPN-NA Top Fives Amended 9-29-16* Young Horse Classes Foals/Weanlings: Dressage Foals/Weanlings: Hunter North American Champion North American Champion Leliscia SSF Life’s Grand RYU Governor x Eliscia SSF, ster by UB 40 Grand Cru x Sakura Hill Czar by Alla Czar Owner/Breeder: Carroll and Carol Tolman, Owner: Sheila Murphy and Shirley Murphy Shooting Star Farm Breeder: Ryu Equestrian, Sheila Murphy and Shirley Murphy Reserve Champion Foals/Weanlings: Gelder Levanta S North American Champion Charmeur x Evanta III MMW, keur by UB 40 Lennox Owner/Breeder: Dan & Gina Ruediger, Paganini, crown x Adessa, keur by Koss, preferent Sonnenberg Farm, LLC Owner/Breeder: Beverley Hilton 3rd Lolita Zarma TF Sir Gregory x Eerste Zarma TF, ster /IBOP-D by Westpoint Yearlings: Dressage Owner/Breeder: KC Dunn, Timbach Farm North American Champion 4th Luca Kenny G S.E. Franklin x Zancerre, PROK by Sir Sinclair, keur Sir Donnerhall x Bardot S.E., ster by Florencio, keur Owner: Miller Stables, LLC Breeder: C.H.I. Huisinga-Terllouw Owner: Teresa Crater, Camelot Farm Breeder: Siegi Belz-Fry, Stall Europa 5th Lyric DG El Capone x Woodwind, elite by Contester Reserve Champion Owner/Breeder: DG Bar Breeders, Inc. & Natalie Bryant Kid Rock S.E. Sir Donnerhall II x Volimbria by Contango, preferent Foals/Weanlings: Jumper Owner/Breeder: Siegi Belz-Fry, Stall Europa North American Champion 3rd Karina Sandra TF Last Chapter Fiderbach x Giselle TF stb by Uphill Goodtimes x Nirvana, ster/ preferent by Fleming Owner/Breeder: KC Dunn, Timbach Farm Owner/Breeder: Larry & Kathy Childs, Crooked Post Farm 4th Kalisandra TF Reserve Champion Uno Don Diego x Giasandra TF by Polansky Lady Ada Bloom Owner/Breeder: KC Dunn, Timbach Farm Corland, keur x Feather Bloom by Mr. -

Intern Kommunikation Kring Hållbarhet - En Fallstudie Vid Swedbank Och Länsförsäkringar Bank
Intern kommunikation kring hållbarhet - En fallstudie vid Swedbank och Länsförsäkringar Bank Kandidatuppsats 15 hp Företagsekonomiska institutionen Uppsala universitet HT 2018 Datum för inlämning: 2019-01-18 Oscar Boström Sofia Lundgren Handledare: Göran Nilsson Sammandrag Hållbarhet är aktuellt för den enskilde individen och för företag. Företag kommunicerar sina hållbarhetsarbeten externt på olika sätt men för att effektivisera hållbarhetsarbetet behöver samtliga medarbetare involveras. Genom en tydlig intern- och tvåvägskommunikation kan företag dra större nytta av deras hållbarhetsarbete. Denna kvalitativa undersökning har syftat till att undersöka hur hållbarhetsarbetet kommuniceras internt i Swedbank och Länsförsäkringar Bank samt i vilken utsträckning företagen uppvisar likheter och skillnader. Forskningsfrågorna har besvarats genom intervjuer med personer i chefsbefattning och kundrådgivare. Teorier om intern kommunikation samt kommunikationsmodell ligger till grund för analysen av den empiriska undersökningen. Trots företagens storleksmässiga och strukturella skillnader uppvisar företagen många likheter. En stor skillnad är var i organisationen en hållbarhetsansvarig är stationerad och hur det påverkar medarbetare på kontoren. Ett förekommande problem som visat sig är mängden information som kommuniceras inom företaget vilket chefer är medvetna om och försöker därför sålla och anpassa informationen. Nyckelord: Hållbarhet, Intern kommunikation, Kommunikationskanal, Kommunikationsväg, Effektiv kommunikation, Information Innehållsförteckning -

Still Swingin' Students Hung Up
the bagpipeonline.comBAGPIPE VOL.57 NO.1 SEPTEMBER 8, 2010 Students hung up about hammock Still swingin’ rules pages 2 & 6 COVENANT ON CAMERAby Adrienne Belz premium” on taking care of stu- Ford explained that the theft [email protected] dents. This latest installment follows problems at Covenant are bigger Covenant’s first new security the ScotsCard residential security than one might expect, and other camera system is up and running created a few years ago, the tornado administrators saw the installment with 22 video cameras keeping an and intruder drill system, and more as a proactive measure. eye on campus. evening security guards. “Covenant wants to take reason- Greg Ford, Safety and Security The system cost the school able precautions,” said President Coordinator, said that if nothing $22,000, Ford said, a figure that Niel Nielson. Nielson said he was else he wants the 22 new cameras had several students raising their surprised at the low cost of Cov- to act as a deterrent to theft. Most eyebrows. enant’s system compared to those of the cameras are nestled in nooks “Why are they spending of other schools, saying that he of the ceilings in residential main $22,000 on them when it feels like thought the bill would be five times entrances, but both gymnasium something we don’t need at all?” what it was. entry ways have them, and last week questioned Matthew Higgins, a Before acquiring the system, a few were hung in the Great Hall, junior from Signal Mountain, Tenn. safety and security staff studied how according to Ford. -

10.05 Gravity and Topography of the Terrestrial Planets MA Wieczorek, University Paris Diderot, Paris Cedex 13, France
10.05 Gravity and Topography of the Terrestrial Planets MA Wieczorek, University Paris Diderot, Paris Cedex 13, France ã 2015 Elsevier B.V. All rights reserved. 10.05.1 Introduction 153 10.05.2 Mathematical Preliminaries 154 10.05.2.1 Spherical Harmonics 155 10.05.2.2 Gravity, Potential, and Geoid 156 10.05.3 The Data 158 10.05.3.1 Earth 158 10.05.3.1.1 Topography 158 10.05.3.1.2 Gravity 159 10.05.3.1.3 Spectral analysis 159 10.05.3.2 Venus 161 10.05.3.2.1 Topography 161 10.05.3.2.2 Gravity 162 10.05.3.2.3 Spectral analysis 162 10.05.3.3 Mars 164 10.05.3.3.1 Topography 164 10.05.3.3.2 Gravity 166 10.05.3.3.3 Spectral analysis 166 10.05.3.4 Mercury 167 10.05.3.4.1 Topography 167 10.05.3.4.2 Gravity 167 10.05.3.4.3 Spectral analysis 169 10.05.3.5 The Moon 169 10.05.3.5.1 Topography 169 10.05.3.5.2 Gravity 170 10.05.3.5.3 Spectral analysis 170 10.05.4 Methods for Calculating Gravity from Topography 172 10.05.5 Crustal Thickness Modeling 173 10.05.6 Admittance Modeling 174 10.05.6.1 Spatial Domain 174 10.05.6.2 Spectral Domain 177 10.05.7 Localized Spectral Analysis 179 10.05.7.1 Multitaper Spectral Analysis 179 10.05.7.2 Wavelet Analysis 180 10.05.8 Summary of Major Results 181 10.05.8.1 Earth 181 10.05.8.2 Venus 182 10.05.8.3 Mars 183 10.05.8.4 Mercury 184 10.05.8.5 The Moon 185 10.05.9 Future Developments and Concluding Remarks 186 Acknowledgments 187 References 187 10.05.1 Introduction means exist to probe the interior structure of the terrestrial planets from orbit. -
Building Permits Issued June 2020(PDF)
BUILDING PERMITS ISSUED Permits Issued June, 2020 PERMIT Issued Description Scope APN Address Valuation Contractor 527643 06/30/2020 WOOD STOVE CHANGEOUT Miscellaneous FAC 085-176-001-000 4325 POPLAR AV HOMEWOOD $3,000.00 MOUNTAIN HOME CENTER BLD18-05034 06/29/2020 Santana - Generator Generator 084-060-002-000 635 WARD AVE TAHOE CITY $10,000.00 BRIAN LEE - CONSTRUCTION BLD18-05844 06/26/2020 GROECHEL - SFD Single Family > 3000 069-490-004-000 1019 SERENE RD SODA SPRINGS $799,650.68 Jaborski Construction BLD18-05845 06/26/2020 GROECHEL - SECONDARY Secondary Dwelling 069-490-004-000 1021 SERENE RD SODA SPRINGS $165,082.90 JABORSKI CONSTRUCTION INC DWELLING BLD19-01297 06/26/2020 PRIOLO- NEW SFD Single Family > 3000 107-180-008-000 10665 CARSON RANGE RD TRUCKEE $644,843.77 WILLIAMS CONSTRUCTION AND DESIGN GROUP INC BLD19-01454 06/26/2020 Groechel - Tower Residential Accessory Other 069-490-004-000 1019 SERENE RD SODA SPRINGS $9,694.74 JABORSKI CONSTRUCTION INC BLD19-02209 06/08/2020 Spindle Shanks-TI Restaurant 117-200-054-000 400 BRASSIE AVE TAHOE VISTA $31,617.55 BLD19-02444 06/16/2020 HUDSON DECK Wood Deck > 30 inch Above Grade 035-290-041-000 6451 VIREO WY GRANITE BAY $9,934.32 BLD19-02756 06/24/2020 TACOS JALISCO TI Restaurant 090-192-004-000 8717 LAKE BLVD KINGS BEACH $31,201.05 CKS INC BLD19-03516 06/30/2020 BURROWS - SPA, FIREPIT Residential Accessory Other 109-280-005-000 7765 LAHONTAN DR TRUCKEE $12,235.60 MIKE DONICA TILE AND CONSTRUCTION INC PATIO ADDITION BLD19-03989 06/08/2020 AUBURN INTERFAITH FOOD Warehouse 054-181-037-000 -

Origin of the Solar System 49 5.1
Introduction to Planetary Science Introduction to Planetary Science The Geological Perspective GUNTER FAURE The Ohio State University, Columbus, Ohio, USA TERESA M. MENSING The Ohio State University, Marion, Ohio, USA A C.I.P. Catalogue record for this book is available from the Library of Congress. ISBN-13 978-1-4020-5233-0 (HB) ISBN-13 978-1-4020-5544-7 (e-book) Published by Springer, P.O. Box 17, 3300 AA Dordrecht, The Netherlands. www.springer.com Cover art: The planets of the solar system. Courtesy of NASA. A Manual of Solutions for the end-of-chapter problems can be found at the book’s homepage at www.springer.com Printed on acid-free paper All Rights Reserved © 2007 Springer No part of this work may be reproduced, stored in a retrieval system, or transmitted in any form or by any means, electronic, mechanical, photocopying, microfilming, recording or otherwise, without written permission from the Publisher, with the exception of any material supplied specifically for the purpose of being entered and executed on a computer system, for exclusive use by the purchaser of the work. In memory of Dr. Erich Langenberg, David H. Carr, and Dr. Robert J. Uffen who showed me the way. Gunter Faure For Professor Tom Wells and Dr. Phil Boger who taught me to reach for the stars. Teresa M. Mensing Table of Contents Preface xvii 1. The Urge to Explore 1 1.1. The Exploration of Planet Earth 2 1.2. Visionaries and Rocket Scientists 4 1.3. Principles of Rocketry and Space Navigation 7 1.4. -
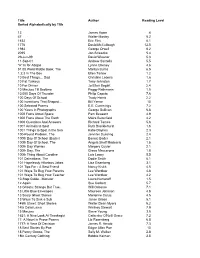
Reading Counts
Title Author Reading Level Sorted Alphabetically by Title 13 James Howe 4 47 Walter Mosley 5.2 1632 Eric Flint 8.1 1776 David McCullough 12.5 1984 George Orwell 8.2 2095 Jon Scieszka 5.4 29-Jun-99 David Wiesner 5.3 11-Sep-01 Andrew Santella 5.5 "A" Is for Abigail Lynne Cheney 4.6 $1.00 Word Riddle Book, The Marilyn Burns 6.5 1,2,3 In The Box Ellen Tarlow 1.2 10 Best Things… Dad Christine Loomis 1.6 10 Fat Turkeys Tony Johnston 1.7 10 For Dinner Jo Ellen Bogart 2.4 10 Minutes Till Bedtime Peggy Rathmann 1.5 10,000 Days Of Thunder Philip Caputo 7.6 100 Days Of School Trudy Harris 2.2 100 Inventions That Shaped... Bill Yenne 10 100 Selected Poems E.E. Cummings 7.2 100 Years In Photographs George Sullivan 6.8 1000 Facts About Space Pam Beasant 4.9 1000 Facts About The Earth Moira Butterfield 4.2 1000 Questions And Answers Richard Tames 5.6 1001 Animals to Spot Ruth Brocklehurst 1.6 1001 Things to Spot in the Sea Katie Daynes 2.3 100-Pound Problem, The Jennifer Dussling 2.4 100th Day Of School (Bader) Bonnie Bader 2.1 100th Day Of School, The Angela Shelf Medearis 1.5 100th Day Worries Margery Cuyler 2.1 100th Day, The Grace Maccarone 1.8 100th Thing About Caroline Lois Lowry 5.5 101 Dalmatians, The Dodie Smith 6.1 101 Hopelessly Hilarious Jokes Lisa Eisenberg 3.1 101 Tips For - A Best Friend Nancy Krulik 4.5 101 Ways To Bug Your Parents Lee Wardlaw 4.8 101 Ways To Bug Your Teacher Lee Wardlaw 4.2 10-Step Guide...Monster Laura Numeroff 1.5 12 Again Sue Corbett 5.7 13 Ghosts: Strange But True..