Genetics of Cattle Pr
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

CATAIR Appendix
CBP and Trade Automated Interface Requirements Appendix: PGA April 24, 2020 Pub # 0875-0419 Contents Table of Changes ............................................................................................................................................4 PG01 – Agency Program Codes .................................................................................................................... 18 PG01 – Government Agency Processing Codes ............................................................................................. 22 PG01 – Electronic Image Submitted Codes.................................................................................................... 26 PG01 – Globally Unique Product Identification Code Qualifiers .................................................................... 26 PG01 – Correction Indicators* ...................................................................................................................... 26 PG02 – Product Code Qualifiers.................................................................................................................... 28 PG04 – Units of Measure .............................................................................................................................. 30 PG05 – Scie nt if ic Spec ies Code .................................................................................................................... 31 PG05 – FWS Wildlife Description Codes ..................................................................................................... -

ATIC1294 {By Email}
Animal and Plant Health Agency F 01932 357608 Access to Information Team Weybourne Building Ground Floor www.gov.uk/apha Woodham Lane New Haw Addlestone Surrey KT15 3NB Our Ref: ATIC1294 {By Email} 17 April 2018 Dear PROVISION OF REQUESTED INFORMATION Thank you for your request for information about bovine Tb in rare breeds which we received on 18 March 2018. Your request has been handled under the Freedom of Information Act 2000 (FOIA) The information you requested and our response is detailed below: ‘I am looking into the impact bovine TB has on traditional/rare breeds of cattle in the United Kingdom. I should be very grateful if you could provide me with the following information:’ ‘Number of cattle of the breeds listed below (pure bred) compulsorily slaughtered as reactors or direct contacts in 2016 and 2017 and if possible, listed by region’ 1) Beef Shorthorn Breed code BSH 2) Whitebred Shorthorn " WS 3) British White " BW 4) Devon " DEV 5) Dexter " DEX 6) Gloucester " GL 7) Guernsey " GU 8) Hereford " HE 9) Jersey " JE 10) Lincoln Red " LR 11) Red Poll " RP 12) South Devon " SD 13) Sussex " SU 14) Welsh Black " WB 15) White Park " WP 16) English Longhorn " LH The Animal and Plant Health Agency is an Executive Agency of the Department for Environment, Food and Rural Affairs working to safeguard animal and plant health for the benefit of people, the environment and the economy. The information you have requested has been placed on a spreadsheet and attached to our reply as Appendix 1. N.B. -

"First Report on the State of the World's Animal Genetic Resources"
Country Report of Australia for the FAO First Report on the State of the World’s Animal Genetic Resources 2 EXECUTIVE SUMMARY................................................................................................................5 CHAPTER 1 ASSESSING THE STATE OF AGRICULTURAL BIODIVERSITY THE FARM ANIMAL SECTOR IN AUSTRALIA.................................................................................7 1.1 OVERVIEW OF AUSTRALIAN AGRICULTURE, ANIMAL PRODUCTION SYSTEMS AND RELATED ANIMAL BIOLOGICAL DIVERSITY. ......................................................................................................7 Australian Agriculture - general context .....................................................................................7 Australia's agricultural sector: production systems, diversity and outputs.................................8 Australian livestock production ...................................................................................................9 1.2 ASSESSING THE STATE OF CONSERVATION OF FARM ANIMAL BIOLOGICAL DIVERSITY..............10 Major agricultural species in Australia.....................................................................................10 Conservation status of important agricultural species in Australia..........................................11 Characterisation and information systems ................................................................................12 1.3 ASSESSING THE STATE OF UTILISATION OF FARM ANIMAL GENETIC RESOURCES IN AUSTRALIA. ........................................................................................................................................................12 -

I . the Color Gene C
THE ABC OF COLOR INHERITANCE IN HORSES W. E. CASTLE Division of Genetics, University of California, Berkeley, California Received October, 27, 1947 HE study of color inheritance in horses was begun in the early days of Tgenetics. Indeed many facts concerning it had already been established earlier, by DARWINin his book on “Variation of Animals and Plants under Domestication.” At irregular inteivals since then, new attempts have been made to collect and classify in terms of genetic factors the records contained in stud books concerning the colors of colts in relation to the colors of their sires and dams. A full bibliography is given by CREWand BuCHANAN-SMITH (19301. By such studies, we have acquired very full information as to what color a colt may be expected to have, when the color of its parents and grandparents is known. This knowledge is empirical rather than experimental in nature. For horses being slow breeding and expensive are rarely available for direct experi- mental study, such as can be made with the small laboratory mammals, mice, rats, rabbits and guinea pigs. We have definite information that color inheritance in horses involves the existence of mutant genes similar to those demonstrated by experimental studies to be involved in color inheritance of other mammals. But the horse genes have been given special names, as they were successively discovered, and it is difficult at present to correlate them with the better known names and geneic symbols used by the experimental breeders. The present paper is an attempt to make such a correlation. Just as in morphological studies comparative anatomy was found useful and still is used to establish homologies between systems of organs, so in mammalian genetics, a comparative study of gene action in the production of coat colors and color patterns may also be of value. -

Use of Genomic Tools to Discover the Cause of Champagne Dilution Coat Color in Horses and to Map the Genetic Cause of Extreme Lordosis in American Saddlebred Horses
University of Kentucky UKnowledge Theses and Dissertations--Veterinary Science Veterinary Science 2014 USE OF GENOMIC TOOLS TO DISCOVER THE CAUSE OF CHAMPAGNE DILUTION COAT COLOR IN HORSES AND TO MAP THE GENETIC CAUSE OF EXTREME LORDOSIS IN AMERICAN SADDLEBRED HORSES Deborah G. Cook University of Kentucky, [email protected] Right click to open a feedback form in a new tab to let us know how this document benefits ou.y Recommended Citation Cook, Deborah G., "USE OF GENOMIC TOOLS TO DISCOVER THE CAUSE OF CHAMPAGNE DILUTION COAT COLOR IN HORSES AND TO MAP THE GENETIC CAUSE OF EXTREME LORDOSIS IN AMERICAN SADDLEBRED HORSES" (2014). Theses and Dissertations--Veterinary Science. 15. https://uknowledge.uky.edu/gluck_etds/15 This Doctoral Dissertation is brought to you for free and open access by the Veterinary Science at UKnowledge. It has been accepted for inclusion in Theses and Dissertations--Veterinary Science by an authorized administrator of UKnowledge. For more information, please contact [email protected]. STUDENT AGREEMENT: I represent that my thesis or dissertation and abstract are my original work. Proper attribution has been given to all outside sources. I understand that I am solely responsible for obtaining any needed copyright permissions. I have obtained needed written permission statement(s) from the owner(s) of each third-party copyrighted matter to be included in my work, allowing electronic distribution (if such use is not permitted by the fair use doctrine) which will be submitted to UKnowledge as Additional File. I hereby grant to The University of Kentucky and its agents the irrevocable, non-exclusive, and royalty-free license to archive and make accessible my work in whole or in part in all forms of media, now or hereafter known. -

Harmful Mutational Load in the Mitogenomes of Cattle Breeds
Harmful mutational load in the mitogenomes of cattle breeds Sankar Subramanian GeneCology Research Centre, School of Science and Engineering, The University of the Sunshine Coast, Moreton Bay, QLD 4502, Australia Supporting Information Table S1. List of Mitochondrial genomes used in the study Accession Accession Accession no. Breed no. Breed no. Breed AY676862 Angus-X MN200832 Brahman JN817322 Domiaty AY676863 Angus-X MN200833 Brahman JN817323 Domiaty AY676864 Angus-X MN200834 Brahman JN817324 Domiaty AY676865 Angus-X MN200836 Brahman KT184452 Domiaty AY676866 Angus-X MN200889 Brahman KT184456 Domiaty AY676867 Angus-X EU177840 Cabannina KT184457 Domiaty AY676868 Angus-X EU177850 Cabannina KT184460 Domiaty AY676869 Angus-X EU177851 Cabannina KT184462 Domiaty AY676870 Angus-X EU177866 Cabannina KT184463 Domiaty AY676871 Angus-X EU177867 Cabannina KT184469 Domiaty AY676872 Angus-X HQ184038 Cabannina KT184470 Domiaty AY676873 Angus-X EU177816 Chianina KT184471 Domiaty DQ124387 Beef EU177818 Chianina KT184472 Domiaty DQ124388 Beef EU177819 Chianina DQ124404 Holstein-Friesian DQ124390 Beef EU177820 Chianina DQ124405 Holstein-Friesian DQ124391 Beef EU177822 Chianina DQ124406 Holstein-Friesian DQ124392 Beef EU177825 Chianina DQ124407 Holstein-Friesian DQ124393 Beef EU177828 Chianina DQ124408 Holstein-Friesian DQ124394 Beef EU177841 Chianina DQ124409 Holstein-Friesian DQ124395 Beef EU177845 Chianina DQ124410 Holstein-Friesian DQ124396 Beef EU177846 Chianina DQ124411 Holstein-Friesian DQ124397 Beef EU177853 Chianina DQ124412 Holstein-Friesian DQ124398 -

Tesi Finale Dottorato
INDICE GENERALE 1. INTRODUZIONE............................................................................................................................3 1.1. La tracciabilità dei prodotti di origine animale: alcuni elementi.........................................3 1.2. Elementi di genetica molecolare..........................................................................................4 1.2.1. I marcatori genetici.......................................................................................................4 1.2.2. Lo stato di avanzamento nello studio del genoma degli animali di interesse zootecnico...............................................................................................................................6 1.3. Tracciabilità dei prodotti di origine animale e genetica molecolare....................................8 1.4. I prodotti “monorazza”.......................................................................................................10 1.5. Genetica e biochimica del colore del mantello: alcuni elementi........................................16 1.6. Genetica molecolare e colore del mantello........................................................................19 1.6.1. Il gene MC1R nella specie bovina.............................................................................21 1.6.2. Il gene MC1R nella specie suina................................................................................26 1.6.3. Il gene KIT nella specie bovina..................................................................................26 -

Multiple Choice Choose the Answer That Best Completes Each Statement Or Question
Name Date Hour 5 The Beef Cattle Industry Multiple Choice Choose the answer that best completes each statement or question. _______ 1. Early settlers primarily used cattle as ____ . A. work animals B. a source of meat C. a source of milk D. symbols of wealth _______ 2. The modern cattle industry is concentrated in the ____ . A. South and Midwest B. South and Southwest C. North and Northwest D. North and Midwest _______ 3. Cattle drives were necessary in the past because of the lack of ____ . A. retail stores B. refrigeration C. slaughterhouses D. year-round grazing _______ 4. Which beef cattle breed is known for its solid black color and excellent meat quality? A. Angus B. Hereford C. Shorthorn D. Chianina _______ 5. Which beef cattle breed is from northern England and was often called a Durham after the county in which it originated? A. Angus B. Hereford C. Shorthorn D. Chianina Introduction to Agriscience | Unit 5 Test CIMC 1 _______ 6. Which beef cattle breed originated in England and was originally much larger, weighing more than 3,000 pounds, than it is today? A. Angus B. Hereford C. Shorthorn D. Chianina _______ 7. Which beef cattle breed is red with a white face and may also have white on the neck, underline, legs, and tail switch? A. Angus B. Hereford C. Shorthorn D. Chianina _______ 8. Which beef cattle breed is one of the oldest breeds in the world and originated in Italy? A. Angus B. Hereford C. Shorthorn D. Chianina _______ 9. Which beef cattle breed originated in central France and was developed as a dual-purpose breed and is typically white or off-white in color? A. -

Revisiting AFLP Fingerprinting for an Unbiased Assessment of Genetic
Utsunomiya et al. BMC Genetics 2014, 15:47 http://www.biomedcentral.com/1471-2156/15/47 RESEARCH ARTICLE Open Access Revisiting AFLP fingerprinting for an unbiased assessment of genetic structure and differentiation of taurine and zebu cattle Yuri Tani Utsunomiya1†, Lorenzo Bomba2†, Giordana Lucente2, Licia Colli2,3, Riccardo Negrini2, Johannes Arjen Lenstra4, Georg Erhardt5, José Fernando Garcia1,6, Paolo Ajmone-Marsan2,3* and European Cattle Genetic Diversity Consortium Abstract Background: Descendants from the extinct aurochs (Bos primigenius), taurine (Bos taurus) and zebu cattle (Bos indicus) were domesticated 10,000 years ago in Southwestern and Southern Asia, respectively, and colonized the world undergoing complex events of admixture and selection. Molecular data, in particular genome-wide single nucleotide polymorphism (SNP) markers, can complement historic and archaeological records to elucidate these past events. However, SNP ascertainment in cattle has been optimized for taurine breeds, imposing limitations to the study of diversity in zebu cattle. As amplified fragment length polymorphism (AFLP) markers are discovered and genotyped as the samples are assayed, this type of marker is free of ascertainment bias. In order to obtain unbiased assessments of genetic differentiation and structure in taurine and zebu cattle, we analyzed a dataset of 135 AFLP markers in 1,593 samples from 13 zebu and 58 taurine breeds, representing nine continental areas. Results: We found a geographical pattern of expected heterozygosity in European taurine breeds decreasing with the distance from the domestication centre, arguing against a large-scale introgression from European or African aurochs. Zebu cattle were found to be at least as diverse as taurine cattle. -

Effect of Breed on Proximate Composition and Fatty Acid Composition of Meat from Italian Cattle
EFFECT OF BREED ON PROXIMATE COMPOSITION AND FATTY ACID COMPOSITION OF MEAT FROM ITALIAN CATTLE Meo Zilio, D. 1, Contò, M. 1, Ballico, S. 1, Ficco A. 1, Failla, S. 1 1 Center for Research and Experimentation in Agriculture, Department of Meat Production and Genetic Improvement (CRA- PCM), Monterotondo Scalo, 00015, Rome, Italy Abstract — Interest in meat fatty acid profile, even though, due to its multiple purpose composition comes from interest in eating aptitude, other specialized beef breed are often healthier meat, i.e. with a higher ratio of preferred by farmers and traders. On the other polyunsaturated (PUFA) to saturated fatty acids hand the use of Holstein male calves for and a more favourable balance between n6 and n3 fattening and meat production is very common. PUFA. The aim of this study was to compare three different bovine genetic types, Maremmana It is characterized by a fast growth and (MM), Chianina (CH) and Italian Holstein (FR) development and produces carcasses and meat on the base of their nutritional fat properties. fatter than typical beef breeds. In particular dairy Meat fatty acid profile was determined by gas breeds deposit more intramuscular fat in relation chromatography analysis. Main fatty acids and to total fat [1]. This characteristic strongly conjugated linoleic acid (CLA), saturated (SFA), influences consumers who prefer meat with monounsatured (MUFA) and polyunsatured good nutritional and organoleptic properties [2]. (PUFA) total were reported. Analysis of obtained From this point of view it is very important to data showed Maremmana breed as a carrier of reduce the saturated fatty acid intake and an some good features in this sense (e.g., low increase of polyunsaturated fatty acids with saturated fatty acid content and high oleic acid contribution) allowing it to play an important role particular regard to n3 (C18:3, C20:5 and in meat cattle breed. -
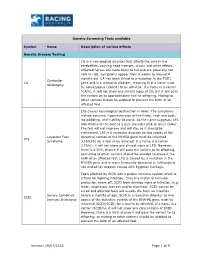
19/07/2018 Page 1 of 9 Genetic Screening Tests Available Symbol
Genetic Screening Tests available Symbol Name Description of variant effects Genetic Disease Testing CA is a neurological disorder that affects the cells in the cerebellum, causing head tremors, ataxia and other effects. Affected horses are more likely to fall and are generally not safe to ride. Symptoms appear from 6 weeks to around 4 months old. CA has been linked to a mutation in the TOE1 Cerebellar CA gene and is a recessive disorder, meaning that a horse must Abiotrophy be homozygous (CA/CA) to be affected. If a horse is a carrier (CA/n), it will not show any clinical signs of CA, but it will pass the variant on to approximately half its offspring. Mating to other carriers should be avoided to prevent the birth of an affected foal. LFS causes neurological dysfunction in foals. The symptoms include seizures, hyperextension of the limbs, neck and back, leg paddling, and inability to stand. As the name suggests, LFS also dilutes to the coat to a pale lavender pink or silver colour. The foal will not improve and will die, so it should be euthanised. LFS is a recessive disorder so two copies of the Lavender Foal defective version of the MYO5A gene must be inherited LFS Syndrome (LFS/LFS) for a foal to be affected. If a horse is a carrier (LFS/n), it will not show any clinical signs of LFS. However, there is a 50% chance it will pass the variant to its offspring, so mating to other carriers should be avoided to prevent the birth of an affected foal. -
Proc1-Beginning Chapters.Pmd
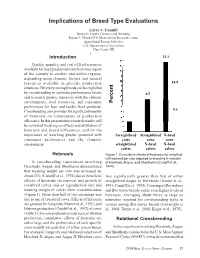
Implications of Breed Type Evaluations Larry V. Cundiff Research Leader, Genetics and Breeding Roman L. Hruska U.S. Meat Animal Research Center Agricultural Research Service U.S. Department of Agriculture Clay Center, NE ()(,) Introduction 23.3 Quality, quantity, and cost of feed resources available for beef production vary from one region of the country to another and within regions, depending upon climatic factors and natural 23.3 resources available in specific production 14.8 situations. Diversity among breeds can be exploited by crossbreeding to optimize performance levels 8.5 and to match genetic resources with the climatic environment, feed resources, and consumer Percent preferences for lean and tender beef products. 8.5 Crossbreeding also provides for significant benefits 14.8 of heterosis on components of production efficiency. In this presentation, research results will be reviewed focusing on effects and utilization of 8.5 8.5 heterosis and breed differences, and on the importance of matching genetic potential with Straightbred Straightbred X-bred consumer preferences and the climatic cows cows cows environment. straightbred X-bred X-bred calves calves calves Heterosis Figure 1. Cumulative effects of heterosis for weight of calf weaned per cow exposed to breeding in crosses A crossbreeding experiment involving of Hereford, Angus, and Shorthorns (Cundiff et al., Herefords, Angus, and Shorthorns demonstrated 1974). that weaning weight per cow was increased by about 23% (Cundiff et al., 1974) due to beneficial was significantly greater than that of either effects of heterosis on survival and growth of straightbred Angus or Herefords (Nunez et al., crossbred calves and on reproduction rate and 1991; Cundiff et al., 1992).