Monocytes Production in Β -Induced IL-1 Δ Kinase C Associated Kinase-1
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Profiling Data
Compound Name DiscoveRx Gene Symbol Entrez Gene Percent Compound Symbol Control Concentration (nM) JNK-IN-8 AAK1 AAK1 69 1000 JNK-IN-8 ABL1(E255K)-phosphorylated ABL1 100 1000 JNK-IN-8 ABL1(F317I)-nonphosphorylated ABL1 87 1000 JNK-IN-8 ABL1(F317I)-phosphorylated ABL1 100 1000 JNK-IN-8 ABL1(F317L)-nonphosphorylated ABL1 65 1000 JNK-IN-8 ABL1(F317L)-phosphorylated ABL1 61 1000 JNK-IN-8 ABL1(H396P)-nonphosphorylated ABL1 42 1000 JNK-IN-8 ABL1(H396P)-phosphorylated ABL1 60 1000 JNK-IN-8 ABL1(M351T)-phosphorylated ABL1 81 1000 JNK-IN-8 ABL1(Q252H)-nonphosphorylated ABL1 100 1000 JNK-IN-8 ABL1(Q252H)-phosphorylated ABL1 56 1000 JNK-IN-8 ABL1(T315I)-nonphosphorylated ABL1 100 1000 JNK-IN-8 ABL1(T315I)-phosphorylated ABL1 92 1000 JNK-IN-8 ABL1(Y253F)-phosphorylated ABL1 71 1000 JNK-IN-8 ABL1-nonphosphorylated ABL1 97 1000 JNK-IN-8 ABL1-phosphorylated ABL1 100 1000 JNK-IN-8 ABL2 ABL2 97 1000 JNK-IN-8 ACVR1 ACVR1 100 1000 JNK-IN-8 ACVR1B ACVR1B 88 1000 JNK-IN-8 ACVR2A ACVR2A 100 1000 JNK-IN-8 ACVR2B ACVR2B 100 1000 JNK-IN-8 ACVRL1 ACVRL1 96 1000 JNK-IN-8 ADCK3 CABC1 100 1000 JNK-IN-8 ADCK4 ADCK4 93 1000 JNK-IN-8 AKT1 AKT1 100 1000 JNK-IN-8 AKT2 AKT2 100 1000 JNK-IN-8 AKT3 AKT3 100 1000 JNK-IN-8 ALK ALK 85 1000 JNK-IN-8 AMPK-alpha1 PRKAA1 100 1000 JNK-IN-8 AMPK-alpha2 PRKAA2 84 1000 JNK-IN-8 ANKK1 ANKK1 75 1000 JNK-IN-8 ARK5 NUAK1 100 1000 JNK-IN-8 ASK1 MAP3K5 100 1000 JNK-IN-8 ASK2 MAP3K6 93 1000 JNK-IN-8 AURKA AURKA 100 1000 JNK-IN-8 AURKA AURKA 84 1000 JNK-IN-8 AURKB AURKB 83 1000 JNK-IN-8 AURKB AURKB 96 1000 JNK-IN-8 AURKC AURKC 95 1000 JNK-IN-8 -

Kinaseseeker™ Full-Length Panel (112 Wild-Type Kinases)
KinaseSeeker™ Full-Length Panel (112 Wild-Type Kinases) Kinase Group Kinase Group ABL1 full-length TK DDR1 intracellular module TK ACVR1 intracellular module TKL DDR2 intracellular module TK AKT1 full-length AGC EGFR intracellular module TK AKT2 full-length AGC EPHA1 intracellular module TK AKT3 full-length AGC EPHA2 intracellular module TK AMPKa1 full-length CAMK EPHA3 intracellular module TK BLK full-length TK EPHA4 intracellular module TK BTK full-length TK EPHA5 intracellular module TK CAMK1D full-length CAMK EPHA6 intracellular module TK CAMK1G full-length CAMK EPHA7 intracellular module TK CAMK2A full-length CAMK EPHA8 intracellular module TK CAMK2B full-length CAMK EPHB3 intracellular module TK CAMK2D full-length CAMK EPHB4 intracellular module TK CAMK2G full-length CAMK ERBB2 intracellular module TK CAMKK1 full-length Other ERBB4 intracellular module TK CAMKK2 full-length Other FAK full-length TK CASK full-length CAMK FGFR2 intracellular module TK CDKL5 full-length CMGC FGFR3 intracellular module TK CK1d full-length CK1 FGR full-length TK CLK1 full-length CMGC FLT1 intracellular module TK CLK2 full-length CMGC FLT2 intracellular module TK CLK3 full-length CMGC FLT4 intracellular module TK CSF1R intracellular module TK FRK full-length TK CSK full-length TK FYN full-length TK DAPK1 full-length CAMK GRK7 full-length AGC Legend: Full-Length: Construct contains Full-length kinase Intracellular Module: Construct contains Cytoplasmic Region in Receptor Tyrosine Kinases Page 1 of 3 KinaseSeeker™ Full-Length Panel (112 Wild-Type Kinases) -

Informationpackage.Pdf
TABLE OF CONTENTS Sample Preparation PDF Page No. 1. Introduction ……………………………………………………………………………………….……. 3 2. Quantity of lysate required …………………………………………………………………………… 5 3. Preparation of cell lysates A. Adherent cells ………………………………………………………………………..…….……. 6 B. Suspension cells ………………………………………………………………………...……… 6 4. Preparation of cell pellets …………………………………………………………………….……… 7 5. Tissue preparation ……………………………………………………………………………………. 7 6. Sample buffer preparation …………………………………………………………………………… 8 Shipping & Pricing 7. Preparation for storage and shipping of samples ……………………………………………..…. 8 8. Shipping information ………………………………………………………………………………….. 8 9. Pricing information ……………………………………………………………………………………. 9 Description of Follow Up Services 10. Follow up services ……………………………………………………………………………………. 9 11. Forms to be completed ………………………………………………………………………………. 10 Sample Buffer Protocol 12. Appendix A - KinetworksTM Sample Buffer protocol ………………………………………………. 15 KinetworksTM Phospho-Site Screening Services 13. Appendix B - List of 38 phosphorylation sites in KPSS-1.3 - Broad Signalling Pathway ….… 16 14. Appendix C - List of 44 phosphorylation sites in KPSS-10.1 - Cell Cycle Status Screen….... 17 15. Appendix D - List of 37 phosphorylation sites in KPSS-11.0 - Protein Kinase Screen …....... 18 16. Appendix E - List of 40 phosphorylation sites in KPSS-12.1 - Substrates of Kinases Screen 19 KinetworksTM Expression Level Profiling Services 17. Appendix F - List of 76 proteins tracked in KPKS-1.2 Screen – Protein Kinase Screen ....… 20 18. Appendix G - List -

IRAK-M Associates with Susceptibility to Adult-Onset Asthma and Promotes Chronic Airway Inflammation
IRAK-M Associates with Susceptibility to Adult-Onset Asthma and Promotes Chronic Airway Inflammation This information is current as Yi Liu, Mingqiang Zhang, Lili Lou, Lun Li, Youming of September 28, 2021. Zhang, Wei Chen, Weixun Zhou, Yan Bai and Jinming Gao J Immunol published online 7 January 2019 http://www.jimmunol.org/content/early/2019/01/04/jimmun ol.1800712 Downloaded from Supplementary http://www.jimmunol.org/content/suppl/2019/01/04/jimmunol.180071 Material 2.DCSupplemental http://www.jimmunol.org/ Why The JI? Submit online. • Rapid Reviews! 30 days* from submission to initial decision • No Triage! Every submission reviewed by practicing scientists • Fast Publication! 4 weeks from acceptance to publication by guest on September 28, 2021 *average Subscription Information about subscribing to The Journal of Immunology is online at: http://jimmunol.org/subscription Permissions Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html Email Alerts Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts The Journal of Immunology is published twice each month by The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright © 2019 by The American Association of Immunologists, Inc. All rights reserved. Print ISSN: 0022-1767 Online ISSN: 1550-6606. Published January 7, 2019, doi:10.4049/jimmunol.1800712 The Journal of Immunology IRAK-M Associates with Susceptibility to Adult-Onset Asthma and Promotes Chronic Airway Inflammation Yi Liu,*,†,1 Mingqiang Zhang,*,1 Lili Lou,* Lun Li,* Youming Zhang,‡ Wei Chen,x Weixun Zhou,{ Yan Bai,‖ and Jinming Gao* IL-1R–associated kinase (IRAK)-M regulates lung immunity during asthmatic airway inflammation. -

Overview of Research on Fusion Genes in Prostate Cancer
2011 Review Article Overview of research on fusion genes in prostate cancer Chunjiao Song1,2, Huan Chen3 1Medical Research Center, Shaoxing People’s Hospital, Shaoxing University School of Medicine, Shaoxing 312000, China; 2Shaoxing Hospital, Zhejiang University School of Medicine, Shaoxing 312000, China; 3Key Laboratory of Microorganism Technology and Bioinformatics Research of Zhejiang Province, Zhejiang Institute of Microbiology, Hangzhou 310000, China Contributions: (I) Conception and design: C Song; (II) Administrative support: Shaoxing Municipal Health and Family Planning Science and Technology Innovation Project (2017CX004) and Shaoxing Public Welfare Applied Research Project (2018C30058); (III) Provision of study materials or patients: None; (IV) Collection and assembly of data: C Song; (V) Data analysis and interpretation: H Chen; (VI) Manuscript writing: All authors; (VII) Final approval of manuscript: All authors. Correspondence to: Chunjiao Song. No. 568 Zhongxing Bei Road, Shaoxing 312000, China. Email: [email protected]. Abstract: Fusion genes are known to drive and promote carcinogenesis and cancer progression. In recent years, the rapid development of biotechnologies has led to the discovery of a large number of fusion genes in prostate cancer specimens. To further investigate them, we summarized the fusion genes. We searched related articles in PubMed, CNKI (Chinese National Knowledge Infrastructure) and other databases, and the data of 92 literatures were summarized after preliminary screening. In this review, we summarized approximated 400 fusion genes since the first specific fusion TMPRSS2-ERG was discovered in prostate cancer in 2005. Some of these are prostate cancer specific, some are high-frequency in the prostate cancer of a certain ethnic group. This is a summary of scientific research in related fields and suggests that some fusion genes may become biomarkers or the targets for individualized therapies. -

Inhibition of ERK 1/2 Kinases Prevents Tendon Matrix Breakdown Ulrich Blache1,2,3, Stefania L
www.nature.com/scientificreports OPEN Inhibition of ERK 1/2 kinases prevents tendon matrix breakdown Ulrich Blache1,2,3, Stefania L. Wunderli1,2,3, Amro A. Hussien1,2, Tino Stauber1,2, Gabriel Flückiger1,2, Maja Bollhalder1,2, Barbara Niederöst1,2, Sandro F. Fucentese1 & Jess G. Snedeker1,2* Tendon extracellular matrix (ECM) mechanical unloading results in tissue degradation and breakdown, with niche-dependent cellular stress directing proteolytic degradation of tendon. Here, we show that the extracellular-signal regulated kinase (ERK) pathway is central in tendon degradation of load-deprived tissue explants. We show that ERK 1/2 are highly phosphorylated in mechanically unloaded tendon fascicles in a vascular niche-dependent manner. Pharmacological inhibition of ERK 1/2 abolishes the induction of ECM catabolic gene expression (MMPs) and fully prevents loss of mechanical properties. Moreover, ERK 1/2 inhibition in unloaded tendon fascicles suppresses features of pathological tissue remodeling such as collagen type 3 matrix switch and the induction of the pro-fbrotic cytokine interleukin 11. This work demonstrates ERK signaling as a central checkpoint to trigger tendon matrix degradation and remodeling using load-deprived tissue explants. Tendon is a musculoskeletal tissue that transmits muscle force to bone. To accomplish its biomechanical function, tendon tissues adopt a specialized extracellular matrix (ECM) structure1. Te load-bearing tendon compart- ment consists of highly aligned collagen-rich fascicles that are interspersed with tendon stromal cells. Tendon is a mechanosensitive tissue whereby physiological mechanical loading is vital for maintaining tendon archi- tecture and homeostasis2. Mechanical unloading of the tissue, for instance following tendon rupture or more localized micro trauma, leads to proteolytic breakdown of the tissue with severe deterioration of both structural and mechanical properties3–5. -

Screen for Kinases Affecting Amyloidogenic Cleavage by BACE1
Screen for kinases affecting amyloidogenic cleavage by BACE1 Dissertation zur Erlangung des akademischen Grades eines Doktors der Naturwissenschaften (Dr. rer. nat.) an der Universität Konstanz Mathematisch-Naturwissenschaftliche Sektion Fachbereich Biologie vorgelegt von Stephan Penzkofer Konstanz, Juli 2011 Tag der mündlichen Prüfung: 24.10.2011 1. Referent: Professor Dr. Marcel Leist 2. Referent: Professor Dr. Daniel Dietrich Summary: The Amyloid β peptide (Aβ) is suspected to be a causal agent for Alzheimer’s disease (AD). Therefore a screen for kinases downregulating the initial step of its production, the cleavage of the Amyloid Precursor Protein (APP) by Beta-site of APP Cleaving Enzyme 1 (BACE1), was conducted in this study. Briefly, HEK293 cells were colipofected with one of in total 1357 siRNAs against 60% of the human kinome and either an APP construct with only the β-cleavage site left or normally cleavable APP as control. Remaining β-cleavage was for logistic reasons firstly measured with an activity-test for secreted alkaline phosphatase (SEAP) fused to both types of APP and subjected to Aβ-ELISA when interesting. Before the screen, the APP-constructs were characterized in the cell types HEK293 and CGCs with regards to cleavage, especially by BACE1. The screen resulted in 38 hits of which one, Testis Specific Serine Kinase 3, was confirmed once more. In a second, bioinformatic project, an initially suspected APLP-like pseudogenic-like sequence in C3orf52 was refuted. Further, analysis of C3orf52 gene expression data hints on a role in myeloid leukemia. Lastly, the phylogenetic relationship of the APP family paralogs was examined, also in comparison to neighboring gene families, and found in the topology (APLP1)(APLP2/APP). -

The Human Gene Connectome As a Map of Short Cuts for Morbid Allele Discovery
The human gene connectome as a map of short cuts for morbid allele discovery Yuval Itana,1, Shen-Ying Zhanga,b, Guillaume Vogta,b, Avinash Abhyankara, Melina Hermana, Patrick Nitschkec, Dror Friedd, Lluis Quintana-Murcie, Laurent Abela,b, and Jean-Laurent Casanovaa,b,f aSt. Giles Laboratory of Human Genetics of Infectious Diseases, Rockefeller Branch, The Rockefeller University, New York, NY 10065; bLaboratory of Human Genetics of Infectious Diseases, Necker Branch, Paris Descartes University, Institut National de la Santé et de la Recherche Médicale U980, Necker Medical School, 75015 Paris, France; cPlateforme Bioinformatique, Université Paris Descartes, 75116 Paris, France; dDepartment of Computer Science, Ben-Gurion University of the Negev, Beer-Sheva 84105, Israel; eUnit of Human Evolutionary Genetics, Centre National de la Recherche Scientifique, Unité de Recherche Associée 3012, Institut Pasteur, F-75015 Paris, France; and fPediatric Immunology-Hematology Unit, Necker Hospital for Sick Children, 75015 Paris, France Edited* by Bruce Beutler, University of Texas Southwestern Medical Center, Dallas, TX, and approved February 15, 2013 (received for review October 19, 2012) High-throughput genomic data reveal thousands of gene variants to detect a single mutated gene, with the other polymorphic genes per patient, and it is often difficult to determine which of these being of less interest. This goes some way to explaining why, variants underlies disease in a given individual. However, at the despite the abundance of NGS data, the discovery of disease- population level, there may be some degree of phenotypic homo- causing alleles from such data remains somewhat limited. geneity, with alterations of specific physiological pathways under- We developed the human gene connectome (HGC) to over- come this problem. -
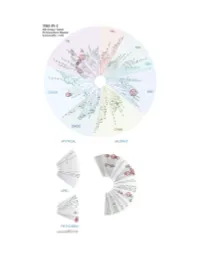
Profiling Data
Entrez Gene Percent Compound Compound Name DiscoveRx Gene Symbol Symbol Control Concentration (nM) THZ-P1-2 AAK1 AAK1 100 1000 THZ-P1-2 ABL1(E255K)-phosphorylated ABL1 21 1000 THZ-P1-2 ABL1(F317I)-nonphosphorylated ABL1 93 1000 THZ-P1-2 ABL1(F317I)-phosphorylated ABL1 100 1000 THZ-P1-2 ABL1(F317L)-nonphosphorylated ABL1 71 1000 THZ-P1-2 ABL1(F317L)-phosphorylated ABL1 44 1000 THZ-P1-2 ABL1(H396P)-nonphosphorylated ABL1 5.8 1000 THZ-P1-2 ABL1(H396P)-phosphorylated ABL1 6.6 1000 THZ-P1-2 ABL1(M351T)-phosphorylated ABL1 12 1000 THZ-P1-2 ABL1(Q252H)-nonphosphorylated ABL1 24 1000 THZ-P1-2 ABL1(Q252H)-phosphorylated ABL1 20 1000 THZ-P1-2 ABL1(T315I)-nonphosphorylated ABL1 93 1000 THZ-P1-2 ABL1(T315I)-phosphorylated ABL1 100 1000 THZ-P1-2 ABL1(Y253F)-phosphorylated ABL1 2.4 1000 THZ-P1-2 ABL1-nonphosphorylated ABL1 13 1000 THZ-P1-2 ABL1-phosphorylated ABL1 8.1 1000 THZ-P1-2 ABL2 ABL2 36 1000 THZ-P1-2 ACVR1 ACVR1 94 1000 THZ-P1-2 ACVR1B ACVR1B 100 1000 THZ-P1-2 ACVR2A ACVR2A 94 1000 THZ-P1-2 ACVR2B ACVR2B 91 1000 THZ-P1-2 ACVRL1 ACVRL1 90 1000 THZ-P1-2 ADCK3 CABC1 77 1000 THZ-P1-2 ADCK4 ADCK4 97 1000 THZ-P1-2 AKT1 AKT1 95 1000 THZ-P1-2 AKT2 AKT2 95 1000 THZ-P1-2 AKT3 AKT3 100 1000 THZ-P1-2 ALK ALK 92 1000 THZ-P1-2 ALK(C1156Y) ALK 93 1000 THZ-P1-2 ALK(L1196M) ALK 71 1000 THZ-P1-2 AMPK-alpha1 PRKAA1 93 1000 THZ-P1-2 AMPK-alpha2 PRKAA2 100 1000 THZ-P1-2 ANKK1 ANKK1 97 1000 THZ-P1-2 ARK5 NUAK1 83 1000 THZ-P1-2 ASK1 MAP3K5 100 1000 THZ-P1-2 ASK2 MAP3K6 95 1000 THZ-P1-2 AURKA AURKA 99 1000 THZ-P1-2 AURKB AURKB 100 1000 THZ-P1-2 AURKC AURKC 83 1000 -

Gene Symbol Accession Alias/Prev Symbol Official Full Name AAK1 NM 014911.2 KIAA1048, Dkfzp686k16132 AP2 Associated Kinase 1
Gene Symbol Accession Alias/Prev Symbol Official Full Name AAK1 NM_014911.2 KIAA1048, DKFZp686K16132 AP2 associated kinase 1 (AAK1) AATK NM_001080395.2 AATYK, AATYK1, KIAA0641, LMR1, LMTK1, p35BP apoptosis-associated tyrosine kinase (AATK) ABL1 NM_007313.2 ABL, JTK7, c-ABL, p150 v-abl Abelson murine leukemia viral oncogene homolog 1 (ABL1) ABL2 NM_007314.3 ABLL, ARG v-abl Abelson murine leukemia viral oncogene homolog 2 (arg, Abelson-related gene) (ABL2) ACVR1 NM_001105.2 ACVRLK2, SKR1, ALK2, ACVR1A activin A receptor ACVR1B NM_004302.3 ACVRLK4, ALK4, SKR2, ActRIB activin A receptor, type IB (ACVR1B) ACVR1C NM_145259.2 ACVRLK7, ALK7 activin A receptor, type IC (ACVR1C) ACVR2A NM_001616.3 ACVR2, ACTRII activin A receptor ACVR2B NM_001106.2 ActR-IIB activin A receptor ACVRL1 NM_000020.1 ACVRLK1, ORW2, HHT2, ALK1, HHT activin A receptor type II-like 1 (ACVRL1) ADCK1 NM_020421.2 FLJ39600 aarF domain containing kinase 1 (ADCK1) ADCK2 NM_052853.3 MGC20727 aarF domain containing kinase 2 (ADCK2) ADCK3 NM_020247.3 CABC1, COQ8, SCAR9 chaperone, ABC1 activity of bc1 complex like (S. pombe) (CABC1) ADCK4 NM_024876.3 aarF domain containing kinase 4 (ADCK4) ADCK5 NM_174922.3 FLJ35454 aarF domain containing kinase 5 (ADCK5) ADRBK1 NM_001619.2 GRK2, BARK1 adrenergic, beta, receptor kinase 1 (ADRBK1) ADRBK2 NM_005160.2 GRK3, BARK2 adrenergic, beta, receptor kinase 2 (ADRBK2) AKT1 NM_001014431.1 RAC, PKB, PRKBA, AKT v-akt murine thymoma viral oncogene homolog 1 (AKT1) AKT2 NM_001626.2 v-akt murine thymoma viral oncogene homolog 2 (AKT2) AKT3 NM_181690.1 -

A Genome-Wide Sirna Screen in Mammalian Cells for Regulators of S6 Phosphorylation
A Genome-Wide siRNA Screen in Mammalian Cells for Regulators of S6 Phosphorylation The Harvard community has made this article openly available. Please share how this access benefits you. Your story matters Citation Papageorgiou, Angela, Joseph Rapley, Jill P. Mesirov, Pablo Tamayo, and Joseph Avruch. 2015. “A Genome-Wide siRNA Screen in Mammalian Cells for Regulators of S6 Phosphorylation.” PLoS ONE 10 (3): e0116096. doi:10.1371/journal.pone.0116096. http:// dx.doi.org/10.1371/journal.pone.0116096. Published Version doi:10.1371/journal.pone.0116096 Citable link http://nrs.harvard.edu/urn-3:HUL.InstRepos:14351232 Terms of Use This article was downloaded from Harvard University’s DASH repository, and is made available under the terms and conditions applicable to Other Posted Material, as set forth at http:// nrs.harvard.edu/urn-3:HUL.InstRepos:dash.current.terms-of- use#LAA RESEARCH ARTICLE A Genome-Wide siRNA Screen in Mammalian Cells for Regulators of S6 Phosphorylation Angela Papageorgiou1,2,3, Joseph Rapley1,2,3, Jill P. Mesirov4, Pablo Tamayo4, Joseph Avruch1,2,3* 1 Department of Molecular Biology, Massachusetts General Hospital, Boston, MA, 02114, United States of America, 2 Diabetes Unit, Medical Services, Massachusetts General Hospital, Boston, MA, 02114, United States of America, 617–726–6909, 3 Department of Medicine, Harvard Medical School, Boston, MA, 02115, United States of America, 4 Broad Institute of MIT and Harvard, 7 Cambridge Center, Cambridge, Massachusetts, 02142, United States of America * [email protected] Abstract mTOR complex1, the major regulator of mRNA translation in all eukaryotic cells, is strongly activated in most cancers. -

A Genome-Wide Sirna Screen in Mammalian Cells for Regulators of S6 Phosphorylation
RESEARCH ARTICLE A Genome-Wide siRNA Screen in Mammalian Cells for Regulators of S6 Phosphorylation Angela Papageorgiou1,2,3, Joseph Rapley1,2,3, Jill P. Mesirov4, Pablo Tamayo4, Joseph Avruch1,2,3* 1 Department of Molecular Biology, Massachusetts General Hospital, Boston, MA, 02114, United States of America, 2 Diabetes Unit, Medical Services, Massachusetts General Hospital, Boston, MA, 02114, United States of America, 617–726–6909, 3 Department of Medicine, Harvard Medical School, Boston, MA, 02115, United States of America, 4 Broad Institute of MIT and Harvard, 7 Cambridge Center, Cambridge, Massachusetts, 02142, United States of America * [email protected] Abstract mTOR complex1, the major regulator of mRNA translation in all eukaryotic cells, is strongly activated in most cancers. We performed a genome-wide RNAi screen in a human cancer cell line, seeking genes that regulate S6 phosphorylation, readout of mTORC1 activity. Ap- plying a stringent selection, we retrieved nearly 600 genes wherein at least two RNAis gave OPEN ACCESS significant reduction in S6-P. This cohort contains known regulators of mTOR complex 1 Citation: Papageorgiou A, Rapley J, Mesirov JP, and is significantly enriched in genes whose depletion affects the proliferation/viability of the Tamayo P, Avruch J (2015) A Genome-Wide siRNA large set of cancer cell lines in the Achilles database in a manner paralleling that caused by Screen in Mammalian Cells for Regulators of S6 Phosphorylation. PLoS ONE 10(3): e0116096. mTOR depletion. We next examined the effect of RNAi pools directed at 534 of these gene doi:10.1371/journal.pone.0116096 products on S6-P in TSC1 null mouse embryo fibroblasts.