Mites from Noctuid Moths
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Lepidoptera of North America 5
Lepidoptera of North America 5. Contributions to the Knowledge of Southern West Virginia Lepidoptera Contributions of the C.P. Gillette Museum of Arthropod Diversity Colorado State University Lepidoptera of North America 5. Contributions to the Knowledge of Southern West Virginia Lepidoptera by Valerio Albu, 1411 E. Sweetbriar Drive Fresno, CA 93720 and Eric Metzler, 1241 Kildale Square North Columbus, OH 43229 April 30, 2004 Contributions of the C.P. Gillette Museum of Arthropod Diversity Colorado State University Cover illustration: Blueberry Sphinx (Paonias astylus (Drury)], an eastern endemic. Photo by Valeriu Albu. ISBN 1084-8819 This publication and others in the series may be ordered from the C.P. Gillette Museum of Arthropod Diversity, Department of Bioagricultural Sciences and Pest Management Colorado State University, Fort Collins, CO 80523 Abstract A list of 1531 species ofLepidoptera is presented, collected over 15 years (1988 to 2002), in eleven southern West Virginia counties. A variety of collecting methods was used, including netting, light attracting, light trapping and pheromone trapping. The specimens were identified by the currently available pictorial sources and determination keys. Many were also sent to specialists for confirmation or identification. The majority of the data was from Kanawha County, reflecting the area of more intensive sampling effort by the senior author. This imbalance of data between Kanawha County and other counties should even out with further sampling of the area. Key Words: Appalachian Mountains, -

Zoogeography of the Holarctic Species of the Noctuidae (Lepidoptera): Importance of the Bering Ian Refuge
© Entomologica Fennica. 8.XI.l991 Zoogeography of the Holarctic species of the Noctuidae (Lepidoptera): importance of the Bering ian refuge Kauri Mikkola, J, D. Lafontaine & V. S. Kononenko Mikkola, K., Lafontaine, J.D. & Kononenko, V. S. 1991 : Zoogeography of the Holarctic species of the Noctuidae (Lepidoptera): importance of the Beringian refuge. - En to mol. Fennica 2: 157- 173. As a result of published and unpublished revisionary work, literature compi lation and expeditions to the Beringian area, 98 species of the Noctuidae are listed as Holarctic and grouped according to their taxonomic and distributional history. Of the 44 species considered to be "naturall y" Holarctic before this study, 27 (61 %) are confirmed as Holarctic; 16 species are added on account of range extensions and 29 because of changes in their taxonomic status; 17 taxa are deleted from the Holarctic list. This brings the total of the group to 72 species. Thirteen species are considered to be introduced by man from Europe, a further eight to have been transported by man in the subtropical areas, and five migrant species, three of them of Neotropical origin, may have been assisted by man. The m~jority of the "naturally" Holarctic species are associated with tundra habitats. The species of dry tundra are frequently endemic to Beringia. In the taiga zone, most Holarctic connections consist of Palaearctic/ Nearctic species pairs. The proportion ofHolarctic species decreases from 100 % in the High Arctic to between 40 and 75 % in Beringia and the northern taiga zone, and from between 10 and 20 % in Newfoundland and Finland to between 2 and 4 % in southern Ontario, Central Europe, Spain and Primorye. -

Zootaxa, Revision of the Micronoctuidae (Lepidoptera
Zootaxa 2583: 1–119 (2010) ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ Monograph ZOOTAXA Copyright © 2010 · Magnolia Press ISSN 1175-5334 (online edition) ZOOTAXA 2583 Revision of the Micronoctuidae (Lepidoptera: Noctuoidea) Part 3, Taxonomy of the Tactusinae MICHAEL FIBIGER Molbechs Allé 49, DK-4180 Sorø, Denmark. E-mail: [email protected] Magnolia Press Auckland, New Zealand Accepted by L. Gall: 16 Jul. 2010; published: 31 Aug. 2010 Michael Fibiger Revision of the Micronoctuidae (Lepidoptera: Noctuoidea) Part 3, Taxonomy of the Tactusinae (Zootaxa 2583) 119 pp.; 30 cm. 31 Aug. 2010 ISBN 978-1-86977-561-2 (paperback) ISBN 978-1-86977-562-9 (Online edition) FIRST PUBLISHED IN 2010 BY Magnolia Press P.O. Box 41-383 Auckland 1346 New Zealand e-mail: [email protected] http://www.mapress.com/zootaxa/ © 2010 Magnolia Press All rights reserved. No part of this publication may be reproduced, stored, transmitted or disseminated, in any form, or by any means, without prior written permission from the publisher, to whom all requests to reproduce copyright material should be directed in writing. This authorization does not extend to any other kind of copying, by any means, in any form, and for any purpose other than private research use. ISSN 1175-5326 (Print edition) ISSN 1175-5334 (Online edition) 2 · Zootaxa 2583 © 2010 Magnolia Press FIBIGER Table of contents Abstract .............................................................................................................................................................................. -

And Lepidoptera Associated with Fraxinus Pennsylvanica Marshall (Oleaceae) in the Red River Valley of Eastern North Dakota
A FAUNAL SURVEY OF COLEOPTERA, HEMIPTERA (HETEROPTERA), AND LEPIDOPTERA ASSOCIATED WITH FRAXINUS PENNSYLVANICA MARSHALL (OLEACEAE) IN THE RED RIVER VALLEY OF EASTERN NORTH DAKOTA A Thesis Submitted to the Graduate Faculty of the North Dakota State University of Agriculture and Applied Science By James Samuel Walker In Partial Fulfillment of the Requirements for the Degree of MASTER OF SCIENCE Major Department: Entomology March 2014 Fargo, North Dakota North Dakota State University Graduate School North DakotaTitle State University North DaGkroadtaua Stet Sacteho Uolniversity A FAUNAL SURVEYG rOFad COLEOPTERA,uate School HEMIPTERA (HETEROPTERA), AND LEPIDOPTERA ASSOCIATED WITH Title A FFRAXINUSAUNAL S UPENNSYLVANICARVEY OF COLEO MARSHALLPTERTAitl,e HEM (OLEACEAE)IPTERA (HET INER THEOPTE REDRA), AND LAE FPAIDUONPATLE RSUAR AVSESYO COIFA CTOEDLE WOIPTTHE RFRAA, XHIENMUISP PTENRNAS (YHLEVTAENRICOAP TMEARRAS),H AANLDL RIVER VALLEY OF EASTERN NORTH DAKOTA L(EOPLIDEAOCPTEEAREA) I ANS TSHOEC RIAETDE RDI VWEITRH V FARLALXEIYN UOSF P EEANSNTSEYRLNV ANNOICRAT HM DAARKSHOATALL (OLEACEAE) IN THE RED RIVER VAL LEY OF EASTERN NORTH DAKOTA ByB y By JAMESJAME SSAMUEL SAMUE LWALKER WALKER JAMES SAMUEL WALKER TheThe Su pSupervisoryervisory C oCommitteemmittee c ecertifiesrtifies t hthatat t hthisis ddisquisition isquisition complies complie swith wit hNorth Nor tDakotah Dako ta State State University’s regulations and meets the accepted standards for the degree of The Supervisory Committee certifies that this disquisition complies with North Dakota State University’s regulations and meets the accepted standards for the degree of University’s regulations and meetMASTERs the acce pOFted SCIENCE standards for the degree of MASTER OF SCIENCE MASTER OF SCIENCE SUPERVISORY COMMITTEE: SUPERVISORY COMMITTEE: SUPERVISORY COMMITTEE: David A. Rider DCoa-CCo-Chairvhiadi rA. -

2010 Season Summary Index NEW WOFTHE~ Zone 1: Yukon Territory
2010 Season Summary Index NEW WOFTHE~ Zone 1: Yukon Territory ........................................................................................... 3 Alaska ... ........................................ ............................................................... 3 LEPIDOPTERISTS Zone 2: British Columbia .................................................... ........................ ............ 6 Idaho .. ... ....................................... ................................................................ 6 Oregon ........ ... .... ........................ .. .. ............................................................ 10 SOCIETY Volume 53 Supplement Sl Washington ................................................................................................ 14 Zone 3: Arizona ............................................................ .................................... ...... 19 The Lepidopterists' Society is a non-profo California ............... ................................................. .............. .. ................... 2 2 educational and scientific organization. The Nevada ..................................................................... ................................ 28 object of the Society, which was formed in Zone 4: Colorado ................................ ... ............... ... ...... ......................................... 2 9 May 1947 and formally constituted in De Montana .................................................................................................... 51 cember -

Kenai National Wildlife Refuge Species List, Version 2018-07-24
Kenai National Wildlife Refuge Species List, version 2018-07-24 Kenai National Wildlife Refuge biology staff July 24, 2018 2 Cover image: map of 16,213 georeferenced occurrence records included in the checklist. Contents Contents 3 Introduction 5 Purpose............................................................ 5 About the list......................................................... 5 Acknowledgments....................................................... 5 Native species 7 Vertebrates .......................................................... 7 Invertebrates ......................................................... 55 Vascular Plants........................................................ 91 Bryophytes ..........................................................164 Other Plants .........................................................171 Chromista...........................................................171 Fungi .............................................................173 Protozoans ..........................................................186 Non-native species 187 Vertebrates ..........................................................187 Invertebrates .........................................................187 Vascular Plants........................................................190 Extirpated species 207 Vertebrates ..........................................................207 Vascular Plants........................................................207 Change log 211 References 213 Index 215 3 Introduction Purpose to avoid implying -
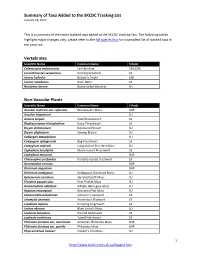
A List of Species New to the SKCDC Tracking List
Summary of Taxa Added to the SKCDC Tracking List January 26, 2017 This is a summary of the newly tracked taxa added to the SKCDC tracking lists. The following tables highlight major changes only; please refer to the full species lists for a complete list of tracked taxa in the province. Vertebrates Scientific Name Common Name S Rank Calamospiza melanocorys Lark Bunting S2B,S2M Coccothraustes vespertinus Evening Grosbeak S4 Icterus bullockii Bullock's Oriole S3B Lontra canadensis River Otter S3 Neotoma cinerea Bushy-tailed Woodrat SH Non-Vascular Plants Scientific Name Common Name S Rank Acaulon muticum var. rufescens Red Acaulon Moss SNR Acaulon triquetrum SU Aneura pinguis Small Greasewort S3 Blepharostoma trichophyllum Hairy Threadwort S3 Bryum dichotomum Bicoloured Bryum SU Bryum uliginosum Swamp Bryum SU Calliergon obtusifolium SU Calypogeia sphagnicola Bog Pouchwort S3 Campylium radicale Long-stalked Fine Wet Moss SU Cephalozia lunulifolia Moon-leaved Pincerwort S3 Cephalozia macounii SNR Chiloscyphus profundus Variable-leaved Crestwort S3 Desmatodon cernuus SNR Dicranum angustum SNR Ditrichum ambiguum Ambiguous Ditrichum Moss SU Ephemerum serratum Serrated Earth Moss SU Fissidens pauperculus Poor Pocket Moss SU Homomallium adnatum Adnate Hairy-gray Moss SU Hypnum recurvatum Recurved Plait Moss SU Jamesoniella autumnalis Jameson's Liverwort S3 Leiomylia anomala Anomalous Flapwort S3 Lepidozia reptans Creeping Fingerwort S3 Leskea obscura Blunt Leske's Moss SU Lophozia longidens Horned Notchwort S3 Lophozia ventricosa Tumid Notchwort -

Moth Records from Burkes Garden, Virginia
14 BANISTERIA NO.2,1993 Banis~ria. Number 2, 1993 iCl 1993 by the Virginia Natural History Society Moth Records from Burkes Garden, Virginia Kenneth J. Stein Department of Entomology Virginia Polytechnic Institute & State University Blacksburg, Virginia 24061 In contrast to butterflies (Clark & Clark, 1951; Covell, and an average annual rainfall of about 119 cm (47 1972), the species composition and distribution of moths inches) (Cooper, 1944). The region was formerly charac have not been well-studied in Virginia. The proceedings terized by oak-chestnut forest, but the chestnut (extirpat of a recent (1989) symposium (Terwilliger, 1991) detailed ed in Virginia) is now replaced largely by hickory. the biological and legal status of numerous plants and Remnants of relict boreal forest persist at elevations animals found in the Commonwealth. Nine species of above 1100 m; Beartown Mountain on the western rim Lepidoptera were reported, with only one moth (Cato retains a vestige of the red spruce forest that occurred cala herodias gerhardi Barnes & Benjamin) designated there prior to intensive lumbering in the early decades as threatened (Schweitzer, in Hoffman, 1991). Covell of this century. Above 1050 m occurs a northern hard (1990) suggested that baseline data are needed to woods forest with a mixture of spruce (Picea rubens understand the diversity and population dynamics of Sarg.), American beech (Fagus grandifolia Erhr.), and both moths and butterflies. He urged that more resourc yellow birch (Betula alleghaniensis Britt) (Woodward & es be appropriated in order to develop regional lepidop Hoffman, 1991). The valley floor has been largely teran checklists, and to learn how best to preserve these deforested and converted into pastureland. -

Michael Fibiger 1945 - 2011
Esperiana Band 16: 7-38 Schwanfeld, 06. Dezember 2011 ISBN 978-3-938249-01-7 Michael FIBIGER 1945 - 2011 Our dear friend and colleague, Michael FIBIGER, died on 16 February, 2011, peacefully and in the presence of the closest members of his family. For close on 18 months he had battled heroically and with characteristic determination against a particularly unpleasant form of cancer, and continued with his writing and research until close to the end. Michael was born on 29 June, 1945, in Hellerup, a suburb of Copenhagen, and began catching moths at the age of nine, particularly in the vicinity of the summer house where they stayed on the north coast of Zealand. By the time he was 11, he wanted to join the Danish Lepidoptera Society but was told he was too young and must wait “a couple of years”. So, exactly two years later he applied again and was accepted – as the youngest-ever Member of the Society. Michael always knew he wanted to be a teacher, and between 1965 and 1970 he attended training college at Hel- lerup Seminarium. Having graduated, he taught Danish, Biology and Special Education at Gentofte School until 1973. In the meantime, he studied Clinical Psychology at the University of Copenhagen from 1970 to 1976, and from 1973 to 1981 he became School Psychologist for elementary schools and high schools in the municipality of Gentofte, work which involved investigation and testing of children with psychiatric problems, counselling, supervi- sion and therapy. He was also an instructor in drug prevention for the Ministry of Education. -

Taxa Names List 6-30-21
Insects and Related Organisms Sorted by Taxa Updated 6/30/21 Order Family Scientific Name Common Name A ACARI Acaridae Acarus siro Linnaeus grain mite ACARI Acaridae Aleuroglyphus ovatus (Troupeau) brownlegged grain mite ACARI Acaridae Rhizoglyphus echinopus (Fumouze & Robin) bulb mite ACARI Acaridae Suidasia nesbitti Hughes scaly grain mite ACARI Acaridae Tyrolichus casei Oudemans cheese mite ACARI Acaridae Tyrophagus putrescentiae (Schrank) mold mite ACARI Analgidae Megninia cubitalis (Mégnin) Feather mite ACARI Argasidae Argas persicus (Oken) Fowl tick ACARI Argasidae Ornithodoros turicata (Dugès) relapsing Fever tick ACARI Argasidae Otobius megnini (Dugès) ear tick ACARI Carpoglyphidae Carpoglyphus lactis (Linnaeus) driedfruit mite ACARI Demodicidae Demodex bovis Stiles cattle Follicle mite ACARI Demodicidae Demodex brevis Bulanova lesser Follicle mite ACARI Demodicidae Demodex canis Leydig dog Follicle mite ACARI Demodicidae Demodex caprae Railliet goat Follicle mite ACARI Demodicidae Demodex cati Mégnin cat Follicle mite ACARI Demodicidae Demodex equi Railliet horse Follicle mite ACARI Demodicidae Demodex folliculorum (Simon) Follicle mite ACARI Demodicidae Demodex ovis Railliet sheep Follicle mite ACARI Demodicidae Demodex phylloides Csokor hog Follicle mite ACARI Dermanyssidae Dermanyssus gallinae (De Geer) chicken mite ACARI Eriophyidae Abacarus hystrix (Nalepa) grain rust mite ACARI Eriophyidae Acalitus essigi (Hassan) redberry mite ACARI Eriophyidae Acalitus gossypii (Banks) cotton blister mite ACARI Eriophyidae Acalitus vaccinii -

Bulletin of the Buffalo Society of Natural Sciences
\\BU/\R\ COMMITTKK ON I'lbLlCATlUX. GEOROE \V. CLINTON, LL. D. GEORGE E. HAYES, D. D. S. W ILMAM H. GLENNY, Ju. LEON F. HARVEY, M. D. GEORGE P. PUTNAM, ^VALTER T. WILSON, AUG. R. GROTE, A. M., CiiAinMAN'. BULLETIN BUFFALO SOCIETY OF NATURAL SCIENCES. LtJ^ — >f BULLETIN BUFFALO SOCIETY OF MTURAL SCIENCES. VOLUME II. From April, 1874, to March, 1875. ^ BUFFALO: PUBLISHED BY THE SOCIETY. 1875. PRESS OP THE COURIER COMPAXV, BUFFALO, N. V. CONTENTS. I. List of the Noctuidae of North America. By Aug. R. Grote, . 1 II. Catalogue of the Coleoptera from the Region of Lake Ponchartrain, La. By S. V. Summers, m. d 78 III. Catalogue of Boleti of New England, with Descriptions of New Species. By Ciias. C. Fkost, 100 IV. On the Species of Helicopis inhabiting the Valley of the Amazon. By Aug. R. Grote, 106 V. Descriptions of New Noctuidae. By H. K. Morrison, • • • • 109 VI. Observations on North American Moths. By Leon F. Harvey, A. M., M. D. 118 VII. Additions to the "List of North American Noctuidae." By Aug. R. Grote, 123 VIII. Land and Fresh Water Shells of the State of New York. By James Lewis, 127 IX. New Noctuae. By Aug. R. Grote 143 X. Notes on American Lepidoptera, with Descriptions of twenty-one New Species. By Aug. R. Gkote, 14.j XI. Determination of the Species of Moths Figured in the " Natural History of New York." By Aug. R. Grote, 1G4 XII. A List of the Leptidae, Mydaidae and Dasypogonina of North America. By Cii. R. OsTEN Sackex 169 XIII. -

(Name) (Degree) (Major) Date Thesis Is Presented
AN ABSTRACT OF THE THESIS OF Anthony Noel McFarland for the M.S. in Entomology (Name) (Degree) ------~~~~~-------(Major) - Date thesis is presented May 10, 1963 ------~--~~-------- Title THE MACROHETEROCERA (LEPIDOPTERA) OF Abstract approved / A continuous twenty-month survey of the Macroheterocera (Lepidoptera) occurring at a location in McDonald Forest, five miles northwest of Corvallis, Benton County, Oregon was conducted. Three hundred sixty species of moths were collected; they repre sented the following families: Sphingidae, Saturniidae, Amatidae, Nolidae, Lithosiidae, Arctiidae, Agaristidae, Noctuidae, Notodonti dae, Liparidae, Lasiocampidae, Thyatiridae, Drepanidae, Geomet ridae, and Epiplemidae. Information is given on the seasonal occur renee, relative abundance, flight habits, and known foodplants of the species collected. Biological and behavioral information is in- eluded for 82 of the species. Comparisons are made between the local fauna and that of the northeastern United States, British Columbia, and a specific locality in southern California. A new device for attracting and holding moths more effec tively within the vicinity of the light (a parabolic moth sheet), which does not involve the use of a trap, is described. THE MACROHETEROCERA (LEPIDOPTERA} OFA MIXED FOREST IN WESTERN OREGON by ANTHONY NOEL McFARLAND A THESIS submitted to OREGON STATE UNIVERSITY in partial fulfillment of the requirements for the degree of MASTER OF SCIENCE June 1963 APPROVED: In Charge of Major Chairman of Department of En Date thesis is presented May l 0, 1963 ----~--~----------- Typed by Jolene Wuest ACKNOWLEDGMENTS I wish to thank the following persons, whose assistance has been of great value: Mr. William R. Bauer and Mr. Steve Buckett of Davis, California, for determination or verification of most of the moths other than Geometrid~e.