B. Tech Chemical Engineering
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Electromagnetic Fields
ELECTROMAGNETIC FIELDS Measure: Why? • Measurement determines whether the • Measurement gives facts. For example, limit values for electromagnetic radiation public protection: On site measurements specified in laws, national regulations, and show the actual exposure levels. This can recommendations are being adhered to. help to allay unfounded anxiety, and can This is essential for occupational safety and be used to initiate further preventive action to protect the general public. – even if the values are well below the permitted limits. • Measurement gives reassurance. It is the most important step in effective protection. • Measurement makes sure. For instance, Take maintenance work on an antenna when planning high tension transmission array, for example: Are the transmitters lines or antenna arrays, spot check measure- switched off? Or is the field strength safe? ments show whether the field strength Do the actual values agree with the theor- simulation used in planning corres ponds to etical ones? reality. MEASURE: WHY? Measure: How? • Only standard-compliant measurements • Non-directional (isotropic) measurement is provide comparable, reproducible, and demanded by the standards. legally defensible results. • The permitted field strengths depend on • The measurement results must be under- the frequency. The measuring instrument standable, even for someone who is not must therefore be sensitive enough, have constantly concerned with electromagnetic a wide dynamic range, and be capable of fields. frequency selective field strength evaluation complying with regulations. • The electric and magnetic fields must be measured separately in the near field. • If the field strength is high or unknown, fit the instrument on a tripod and measure by remote control. MEASURE: HOW? Measure right! • Measurement means comparison with a known quantity. -

Distillation 6
CHAPTER Energy Considerations in Distillation 6 Megan Jobson School of Chemical Engineering and Analytical Science, The University of Manchester, Manchester, UK CHAPTER OUTLINE 6.1 Introduction to energy efficiency ....................................................................... 226 6.1.1 Energy efficiency: technical issues................................................... 227 6.1.1.1 Heating.................................................................................... 227 6.1.1.2 Coolingdabove ambient temperatures...................................... 229 6.1.1.3 Coolingdbelow ambient temperatures ...................................... 230 6.1.1.4 Mechanical or electrical power.................................................. 232 6.1.1.5 Summarydtechnical aspects of energy efficiency ..................... 233 6.1.2 Energy efficiency: process economics............................................... 233 6.1.2.1 Heating.................................................................................... 233 6.1.2.2 Cooling..................................................................................... 235 6.1.2.3 Summarydprocess economics and energy efficiency ............... 237 6.1.3 Energy efficiency: sustainable industrial development........................ 237 6.2 Energy-efficient distillation ............................................................................... 237 6.2.1 Energy-efficient distillation: conceptual design of simple columns...... 238 6.2.1.1 Degrees of freedom in design .................................................. -

(5G) Electromagnetic Field (EMF) Measurement and Maximum Power Extrapolation for Human Exposure Assessment
environments Article Experimental Procedure for Fifth Generation (5G) Electromagnetic Field (EMF) Measurement and Maximum Power Extrapolation for Human Exposure Assessment Daniele Franci 1,*, Stefano Coltellacci 1, Enrico Grillo 1, Settimio Pavoncello 1, Tommaso Aureli 1, Rossana Cintoli 1 and Marco Donald Migliore 2,3 1 ARPA Lazio (Agenzia per la Protezione Ambientale del Lazio), Via G. Saredo 52, 00172 Rome, Italy; [email protected] (S.C.); [email protected] (E.G.); [email protected] (S.P.); [email protected] (T.A.); [email protected] (R.C.) 2 DIEI (Dipartimento di Ingegeria Elettrica e dell’Informazione “Maurizio Scarano”)—University of Cassino and Southern Lazio, via G. Di Biasio 43,03043 Cassino, Italy; [email protected] 3 ICEmB(Inter-University National Research Center on Interactions Between Electromagnetic Fields and Biosystems), Via All’Opera Pia, 11 A, 16145 Genova GE, Italy * Correspondence: [email protected] Received: 30 January 2020; Accepted: 13 March 2020; Published: 17 March 2020 Abstract: The fifth generation (5G) technology has been conceived to cover multiple usage scenarios from enhanced mobile broadband to ultra-reliable low-latency communications (URLLC) to massive machine type communications. However, the implementation of this new technology is causing increasing concern over the possible impact on health and safety arising from exposure to electromagnetic field radiated by 5G systems, making imperative the development of accurate electromagnetic field (EMF) measurement techniques and protocols. Measurement techniques used to assess the compliance with EMF exposure limits are object to international regulation. The basic principle of the assessment is to measure the power received from a constant radio frequency source, typically a pilot signal, and to apply a proper extrapolation factor. -

Review of Extractive Distillation. Process Design, Operation
Review of Extractive Distillation. Process design, operation optimization and control Vincent Gerbaud, Ivonne Rodríguez-Donis, Laszlo Hegely, Péter Láng, Ferenc Dénes, Xinqiang You To cite this version: Vincent Gerbaud, Ivonne Rodríguez-Donis, Laszlo Hegely, Péter Láng, Ferenc Dénes, et al.. Review of Extractive Distillation. Process design, operation optimization and control. Chemical Engineering Research and Design, Elsevier, 2019, 141, pp.229-271. 10.1016/j.cherd.2018.09.020. hal-02161920 HAL Id: hal-02161920 https://hal.archives-ouvertes.fr/hal-02161920 Submitted on 21 Jun 2019 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Open Archive Toulouse Archive Ouverte OATAO is an open access repository that collects the work of Toulouse researchers and makes it freely available over the web where possible This is an author’s version published in: http://oatao.univ-toulouse.fr/239894 Official URL: https://doi.org/10.1016/j.cherd.2018.09.020 To cite this version: Gerbaud, Vincent and Rodríguez-Donis, Ivonne and Hegely, Laszlo and Láng, Péter and Dénes, Ferenc and You, Xinqiang Review of Extractive Distillation. Process design, operation optimization and control. (2018) Chemical Engineering Research and Design, 141. -

Distillation Theory
Chapter 2 Distillation Theory by Ivar J. Halvorsen and Sigurd Skogestad Norwegian University of Science and Technology Department of Chemical Engineering 7491 Trondheim, Norway This is a revised version of an article published in the Encyclopedia of Separation Science by Aca- demic Press Ltd. (2000). The article gives some of the basics of distillation theory and its purpose is to provide basic understanding and some tools for simple hand calculations of distillation col- umns. The methods presented here can be used to obtain simple estimates and to check more rigorous computations. NTNU Dr. ing. Thesis 2001:43 Ivar J. Halvorsen 28 2.1 Introduction Distillation is a very old separation technology for separating liquid mixtures that can be traced back to the chemists in Alexandria in the first century A.D. Today distillation is the most important industrial separation technology. It is particu- larly well suited for high purity separations since any degree of separation can be obtained with a fixed energy consumption by increasing the number of equilib- rium stages. To describe the degree of separation between two components in a column or in a column section, we introduce the separation factor: ()⁄ xL xH S = ------------------------T (2.1) ()x ⁄ x L H B where x denotes mole fraction of a component, subscript L denotes light compo- nent, H heavy component, T denotes the top of the section, and B the bottom. It is relatively straightforward to derive models of distillation columns based on almost any degree of detail, and also to use such models to simulate the behaviour on a computer. -

Distillation Accessscience from McgrawHill Education
6/19/2017 Distillation AccessScience from McGrawHill Education (http://www.accessscience.com/) Distillation Article by: King, C. Judson University of California, Berkeley, California. Last updated: 2014 DOI: https://doi.org/10.1036/10978542.201100 (https://doi.org/10.1036/10978542.201100) Content Hide Simple distillations Fractional distillation Vaporliquid equilibria Distillation pressure Molecular distillation Extractive and azeotropic distillation Enhancing energy efficiency Computational methods Stage efficiency Links to Primary Literature Additional Readings A method for separating homogeneous mixtures based upon equilibration of liquid and vapor phases. Substances that differ in volatility appear in different proportions in vapor and liquid phases at equilibrium with one another. Thus, vaporizing part of a volatile liquid produces vapor and liquid products that differ in composition. This outcome constitutes a separation among the components in the original liquid. Through appropriate configurations of repeated vaporliquid contactings, the degree of separation among components differing in volatility can be increased manyfold. See also: Phase equilibrium (/content/phaseequilibrium/505500) Distillation is by far the most common method of separation in the petroleum, natural gas, and petrochemical industries. Its many applications in other industries include air fractionation, solvent recovery and recycling, separation of light isotopes such as hydrogen and deuterium, and production of alcoholic beverages, flavors, fatty acids, and food oils. Simple distillations The two most elementary forms of distillation are a continuous equilibrium distillation and a simple batch distillation (Fig. 1). http://www.accessscience.com/content/distillation/201100 1/10 6/19/2017 Distillation AccessScience from McGrawHill Education Fig. 1 Simple distillations. (a) Continuous equilibrium distillation. -

Computer Simulation Applied to Studying Continuous Spirit Distillation and Product Quality Control
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector Food Control 22 (2011) 1592e1603 Contents lists available at ScienceDirect Food Control journal homepage: www.elsevier.com/locate/foodcont Computer simulation applied to studying continuous spirit distillation and product quality control Fabio R.M. Batista, Antonio J.A. Meirelles* Laboratory EXTRAE, Department of Food Engineering, Faculty of Food Engineering, University of Campinas e UNICAMP, Campinas, Brazil article info abstract Article history: This work aims to study continuous spirit distillation by computational simulation, presenting some Received 7 June 2010 strategies of process control to regulate the volatile content. The commercial simulator Aspen (Plus and Received in revised form dynamics) was selected. A standard solution containing ethanol, water and 10 minor components rep- 2 March 2011 resented the wine to be distilled. A careful investigation of the vaporeliquid equilibrium was performed Accepted 8 March 2011 for the simulation of two different industrial plants. The simulation procedure was validated against experimental results collected from an industrial plant for bioethanol distillation. The simulations were Keywords: conducted with and without the presence of a degassing system, in order to evaluate the efficiency of Spirits fi Distillation this system in the control of the volatile content. To improve the ef ciency of the degassing system, fl Simulation a control loop based on a feedback controller was developed. The results showed that re ux ratio and Aspen Plus product flow rate have an important influence on the spirit composition. High reflux ratios and spirit Degassing flow rates allow for better control of spirit contamination. -

Yntbietic Fnei
23c$b/J jflt yntbietic fnei OIL SHALE 0 COAL 0 OIL SANDS VOLUME 24 — NUMBER 3 — SEPTEMBER 1987 QUARTERLY Toll Eril Reposito,y ,.r;.'.ur Lakas Library csdo School of Mcs © THE PACE CONSULTANTS INC. 0 Reg. U.S. Pal. OFF. Pace Synthetic Fuels Report Is published by The Pace Consultants Inc., as a multi-client service and is intended for the sole use of the clients or organizations affiliated with clients by virtue of a relationship equivalent to 51 percent or greater ownership. Pace Synthetic Fuels Report is protected by the copyright laws of the United States; reproduction of any part of the publication requires the express permission of The Pace Con- sultants Inc. The Pace Consultants Inc., has provided energy consulting and engineering services since 1955. The company's experience includes resource evalua- tion, process development and design, systems planning, marketing studies, licenser comparisons, environmental planning, and economic analysis. The Synthetic Fuels Analysis group prepares a variety of periodic and other reports analyzing developments in the energy field. THE PACE CONSULTANTS INC. SYNTHETIC FUELS ANALYSIS MANAGING EDITOR Jerry E. Sinor Pt Office Box 649 Niwot, Colorado 80544 (303) 652-2632 BUSINESS MANAGER Ronald L. Gist Post Office Box 53473 Houston, Texas 77052 (713) 669-8800 Telex: 77-4350 CONTENTS HIGHLIGHTS A-i I. GENERAL CORPORATIONS Gill Cuts Coal Gasification Programs 1-i API Data Shows Drop in Oil Production 1-4 GOVERNMENT Petroleum Research Institute Proposed i-S FERC Kills Incremental Gas Pricing 1-5 California to Fund Advanced Energy Technologies i_s ERAB Studying Energy Competitiveness 1-6 ENERGY POLICY AND FORECASTS U.S. -

Green Petroleum Refining - Mathematical Models for Optimizing Petroleum Refining Under Emission Constraints
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by University of Waterloo's Institutional Repository Green Petroleum Refining - Mathematical Models for Optimizing Petroleum Refining Under Emission Constraints by Yusuf Ali Yusuf A thesis presented to the University of Waterloo in fulfillment of the thesis requirement for the degree of Master of Applied Science in Chemical Engineering Waterloo, Ontario, Canada, 2013 © Yusuf Ali Yusuf 2013 I hereby declare that I am the sole author of this thesis. This is a true copy of the thesis, including any required final revisions, as accepted by my examiners. I understand that my thesis may be made electronically available to the public. ii Abstract Petroleum refining processes provide the daily requirements of energy for the global market. Each refining process produces wastes that have the capacity to harm the environment if not properly disposed of. The treatment of refinery waste is one of the most complex issues faced by refinery managers. Also, the hazardous nature of these wastes makes them rather costly to dispose of for the refineries. In this thesis, system analysis tools are used to design a program that allows for the selection of the optimal control, minimization and treating options for petroleum refinery waste streams. The performance of the developed model is demonstrated via a case study. Optimal mitigation alternatives to meet the emission reduction targets were studied by evaluating their relative impact on the profitable operation of the given facility. It was found that the optimal mitigation steps was to reduce emission precursors by conducting feed switches at the refinery. -

CALCULUS for the UTTERLY CONFUSED Has Proven to Be a Wonderful Review Enabling Me to Move Forward in Application of Calculus and Advanced Topics in Mathematics
TLFeBOOK WHAT READERS ARE SAYIN6 "I wish I had had this book when I needed it most, which was during my pre-med classes. It could have also been a great tool for me in a few medical school courses." Or. Kellie Aosley8 Recent Hedical school &a&ate "CALCULUS FOR THE UTTERLY CONFUSED has proven to be a wonderful review enabling me to move forward in application of calculus and advanced topics in mathematics. I found it easy to use and great as a reference for those darker aspects of calculus. I' Aaron Ladeville, Ekyiheeriky Student 'I1 am so thankful for CALCULUS FOR THE UTTERLY CONFUSED! I started out Clueless but ended with an All' Erika Dickstein8 0usihess school Student "As a non-traditional student one thing I have learned is the importance of material supplementary to texts. Especially in calculus it helps to have a second source, especially one as lucid and fun to read as CALCULUS FOR THE UTTERtY CONFUSED. Anyone, whether you are a math weenie or not, will get something out of this book. With this book, your chances of survival in the calculus jungle are greatly increased.'I Brad &3~ker,Physics Student Other books in the Utterly Conhrsed Series include: Financial Planning for the Utterly Confrcsed, Fifth Edition Job Hunting for the Utterly Confrcred Physics for the Utterly Confrred CALCULUS FOR THE UTTERLY CONFUSED Robert M. Oman Daniel M. Oman McGraw -Hill New York San Francisco Washington, D.C. Auckland Bogoth Caracas Lisbon London Madrid Mexico City Milan Montreal New Delhi San Juan Singapore Sydney Tokyo Toronto Library of Congress Cataloging-in-Publication Data Oman, Robert M. -

Impeller Power Draw Across the Full Reynolds Number
IMPELLER POWER DRAW ACROSS THE FULL REYNOLDS NUMBER SPECTRUM Thesis Submitted to The School of Engineering of the UNIVERSITY OF DAYTON In Partial Fulfillment of the Requirements for The Degree of Master of Science in Chemical Engineering By Zheng Ma Dayton, OH August, 2014 IMPELLER POWER DRAW ACROSS THE FULL REYNOLDS NUMBER SPECTRUM Name: Ma, Zheng APPROVED BY: ______________________ ________________________ Kevin J. Myers, D.Sc., P.E. Eric E. Janz, P.E. Advisory Committee Chairman Research Advisor Research Advisor & Professor Chemineer Research & Chemical & Materials Engineering Development Manager NOV-Process & Flow Technologies ________________________ Robert J. Wilkens, Ph.D., P.E. Committee Member & Professor Chemical & Materials Engineering _______________________ ___________________________ John G. Weber, Ph.D. Eddy M. Rojas, Ph.D., M.A., P.E. Associate Dean Dean, School of Engineering School of Engineering ii ABSTRACT IMPELLER POWER DRAW ACROSS THE FULL REYNOLDS NUMBER SPECTRUM Name: Ma, Zheng University of Dayton Research Advisors: Dr. Kevin J. Myers Eric E. Janz The objective of this work is to gain information that could be used to design full scale mixing systems, and also could develop a design guide that can provide a reliable prediction of the power draw of different types of impellers. To achieve this goal, the power number behavior,including three operation regimes, the limits of the operation regimes, and the effect of baffling on power number,was compared across the full Reynolds number spectrum for Newtonian fluids in a laboratory-scale agitator. Six industrially significant impellers were tested, including three radial flow impellers: D-6, CD-6, and S-4, and also three axial flow impellers: P-4, SC-3, and HE-3. -
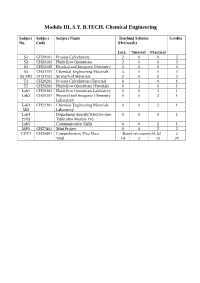
Module III, S.Y. B.TECH. Chemical Engineering
Module III, S.Y. B.TECH. Chemical Engineering Subject Subject Subject Name Teaching Scheme Credits No. Code (Hrs/week) Lect. Tutorial Practical S1 CH20101 Process Calculations 3 0 0 3 S2 CH20103 Fluid-flow Operations 3 0 0 3 S3 CH20105 Physical and Inorganic Chemistry 3 0 0 3 S4 CH21101 Chemical Engineering Materials 3 0 0 3 S5 MD CH21103 Strength of Materials 2 0 0 2 T1 CH20201 Process Calculations (Tutorial) 0 1 0 1 T2 CH20203 Fluid-flow Operations (Tutorial) 0 1 0 1 Lab1 CH20303 Fluid-flow Operations Laboratory 0 0 2 1 Lab2 CH20307 Physical and Inorganic Chemistry 0 0 2 1 Laboratory Lab3 CH21301 Chemical Engineering Materials 0 0 2 1 MD Laboratory Lab4 Department Specific Elective (see 0 0 2 1 (SD) Table after Module IV) Lab5 Communication Skills 0 0 2 1 MP3 CH27401 Mini Project 0 0 2 2 CVV1 CH20401 Comprehensive Viva Voce Based on courses S1,S2 2 Total 14 2 12 25 Issue 05: Rev No. 1: Dt. 30/03/15 FF No: 654A CH 20101:: PROCESS CALCULATIONS Credits: 03 Teaching Scheme: 3 Hours / Week Unit I: Basic Chemical Calculations (8 Hrs) A. Dimensions and Units, chemical calculations including mole, equivalent weight, solids, liquids, solutions and their properties, properties of gases. B. Significance Unit conversions of mass, energy and pressure Unit II: Material Balances Without Chemical Reactions (8 Hrs) A. Process flow sheet, Concept, Material balance calculations, recycling and bypassing operations, material balance of unsteady state processes. B. Material balance of unit operations such as distillation, crystallization Unit III: Material Balances Involving Chemical Reactions (8 Hrs) A.