Patterns in the Genome
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Mobile Genetic Elements in Streptococci
Curr. Issues Mol. Biol. (2019) 32: 123-166. DOI: https://dx.doi.org/10.21775/cimb.032.123 Mobile Genetic Elements in Streptococci Miao Lu#, Tao Gong#, Anqi Zhang, Boyu Tang, Jiamin Chen, Zhong Zhang, Yuqing Li*, Xuedong Zhou* State Key Laboratory of Oral Diseases, National Clinical Research Center for Oral Diseases, West China Hospital of Stomatology, Sichuan University, Chengdu, PR China. #Miao Lu and Tao Gong contributed equally to this work. *Address correspondence to: [email protected], [email protected] Abstract Streptococci are a group of Gram-positive bacteria belonging to the family Streptococcaceae, which are responsible of multiple diseases. Some of these species can cause invasive infection that may result in life-threatening illness. Moreover, antibiotic-resistant bacteria are considerably increasing, thus imposing a global consideration. One of the main causes of this resistance is the horizontal gene transfer (HGT), associated to gene transfer agents including transposons, integrons, plasmids and bacteriophages. These agents, which are called mobile genetic elements (MGEs), encode proteins able to mediate DNA movements. This review briefly describes MGEs in streptococci, focusing on their structure and properties related to HGT and antibiotic resistance. caister.com/cimb 123 Curr. Issues Mol. Biol. (2019) Vol. 32 Mobile Genetic Elements Lu et al Introduction Streptococci are a group of Gram-positive bacteria widely distributed across human and animals. Unlike the Staphylococcus species, streptococci are catalase negative and are subclassified into the three subspecies alpha, beta and gamma according to the partial, complete or absent hemolysis induced, respectively. The beta hemolytic streptococci species are further classified by the cell wall carbohydrate composition (Lancefield, 1933) and according to human diseases in Lancefield groups A, B, C and G. -
SM08 Programme
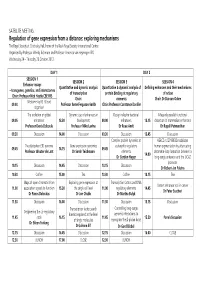
SATELLITE MEETING Regulation of gene expression from a distance: exploring mechanisms The Royal Society at Chicheley Hall, home of the Kavli Royal Society International Centre Organised by Professor Wendy Bickmore and Professor Veronica van Heyningen FRS Wednesday 24 – Thursday 25 October 2012 DAY 1 DAY 2 SESSION 1 SESSION 2 SESSION 3 SESSION 4 Enhancer assays Quantitative and dynamic analysis Quantitative & dynamic analysis of Defining enhancers and their mechanisms – transgenes, genetics, and interactomes of transcription protein binding at regulatory of action Chair: Professor Nick Hastie CBE FRS Chair: elements Chair: Dr Duncan Odom Welcome by RS & lead 09.00 Professor Anne Ferguson-Smith Chair: Professor Constance Bonifer organiser The evolution of global Dynamic use of enhancers in Design rules for bacterial Massively parallel functional 09.05 enhancers 13.30 development 09.00 enhancers 13.15 dissection of mammalian enhancers Professor Denis Duboule Professor Mike Levine Dr Roee Amit Dr Rupali Patwardhan 09.30 Discussion 14.00 Discussion 09.30 Discussion 13.45 Discussion Complex protein dynamics at HERC2 rs12913832 modulates The pluripotent 3D genome Gene expression genomics eukaryotic regulatory human pigmentation by attenuating 09.45 14.15 09.45 Professor Wouter de Laat Dr Sarah Teichmann elements chromatin-loop formation between a 14.00 Dr Gordon Hager long-range enhancer and the OCA2 promoter 10.15 Discussion 14.45 Discussion 10.15 Discussion Dr Robert-Jan Palstra 10.30 Coffee 15.00 Tea 10.30 Coffee 14.15 Tea Maps of open chromatin -

From the Human Genome Project to Genomic Medicine a Journey to Advance Human Health
From the Human Genome Project to Genomic Medicine A Journey to Advance Human Health Eric Green, M.D., Ph.D. Director, NHGRI The Origin of “Genomics”: 1987 Genomics (1987) “For the newly developing discipline of [genome] mapping/sequencing (including the analysis of the information), we have adopted the term GENOMICS… ‘The Genome Institute’ Office for Human Genome Research 1988-1989 National Center for Human Genome Research 1989-1997 National Human Genome Research Institute 1997-present NHGRI: Circa 1990-2003 Human Genome Project NHGRI Today: Characteristic Features . Relatively young (~28 years) . Relatively small (~1.7% of NIH) . Unusual historical origins (think ‘Human Genome Project’) . Emphasis on ‘Team Science’ (think managed ‘consortia’) . Rapidly disseminating footprint (think ‘genomics’) . Novel societal/bioethics research component (think ‘ELSI’) . Over-achievers for trans-NIH initiatives (think ‘Common Fund’) . Vibrant (and large) Intramural Research Program A Quarter Century of Genomics Human Genome Sequenced for First Time by the Human Genome Project Genomic Medicine An emerging medical discipline that involves using genomic information about an individual as part of their clinical care (e.g., for diagnostic or therapeutic decision- making) and the other implications of that clinical use The Path to Genomic Medicine ? Human Realization of Genome Genomic Project Medicine Nature Nature Base Pairs to Bedside 2003 Heli201x to 1Health A Quarter Century of Genomics Human Genome Sequenced for First Time by the Human Genome Project -

Gene Expression Studies: from Case-Control to Multiple-Population-Based Studies
From the Institute of Human Genetics, Helmholtz Zentrum Munchen,¨ Deutsches Forschungszentrum fur¨ Gesundheit und Umwelt (GmbH) Head: Prof. Dr. Thomas Meitinger Gene expression studies: From case-control to multiple-population-based studies Thesis Submitted for a Doctoral Degree in Natural Sciences at the Faculty of Medicine, Ludwig-Maximilians-Universitat¨ Munchen¨ Katharina Schramm Dachau, Germany 2016 With approval of the Faculty of Medicine Ludwig-Maximilians-Universit¨atM ¨unchen Supervisor/Examiner: Prof. Dr. Thomas Illig Co-Examiners: Prof. Dr. Roland Kappler Dean: Prof. Dr. med. dent. Reinhard Hickel Date of oral examination: 22.12.2016 II Dedicated to my family. III Abstract Recent technological developments allow genome-wide scans of gene expression levels. The reduction of costs and increasing parallelization of processing enable the quantification of 47,000 transcripts in up to twelve samples on a single microarray. Thereby the data collec- tion of large population-based studies was improved. During my PhD, I first developed a workflow for the statistical analyses of case-control stu- dies of up to 50 samples. With large population-based data sets generated I established a pipeline for quality control, data preprocessing and correction for confounders, which re- sulted in substantially improved data. In total, I processed more than 3,000 genome-wide expression profiles using the generated pipeline. With 993 whole blood samples from the population-based KORA (Cooperative Health Research in the Region of Augsburg) study we established one of the largest population-based resource. Using this data set we contributed to a number of transcriptome-wide association studies within national (MetaXpress) and international (CHARGE) consortia. -

EMBC Annual Report 2007
EMBO | EMBC annual report 2007 EUROPEAN MOLECULAR BIOLOGY ORGANIZATION | EUROPEAN MOLECULAR BIOLOGY CONFERENCE EMBO | EMBC table of contents introduction preface by Hermann Bujard, EMBO 4 preface by Tim Hunt and Christiane Nüsslein-Volhard, EMBO Council 6 preface by Marja Makarow and Isabella Beretta, EMBC 7 past & present timeline 10 brief history 11 EMBO | EMBC | EMBL aims 12 EMBO actions 2007 15 EMBC actions 2007 17 EMBO & EMBC programmes and activities fellowship programme 20 courses & workshops programme 21 young investigator programme 22 installation grants 23 science & society programme 24 electronic information programme 25 EMBO activities The EMBO Journal 28 EMBO reports 29 Molecular Systems Biology 30 journal subject categories 31 national science reviews 32 women in science 33 gold medal 34 award for communication in the life sciences 35 plenary lectures 36 communications 37 European Life Sciences Forum (ELSF) 38 ➔ 2 table of contents appendix EMBC delegates and advisers 42 EMBC scale of contributions 49 EMBO council members 2007 50 EMBO committee members & auditors 2007 51 EMBO council members 2008 52 EMBO committee members & auditors 2008 53 EMBO members elected in 2007 54 advisory editorial boards & senior editors 2007 64 long-term fellowship awards 2007 66 long-term fellowships: statistics 82 long-term fellowships 2007: geographical distribution 84 short-term fellowship awards 2007 86 short-term fellowships: statistics 104 short-term fellowships 2007: geographical distribution 106 young investigators 2007 108 installation -

A Study of Visitors to Genome: the Secret of How Life Works
A STUDY OF VISITORS TO GENOME: THE SECRET OF HOW LIFE WORKS March 2004 Office of Policy and Analysis Washington, DC 20560-0405 Office of Policy and Analysis Study Team Ioana Munteanu Andrew Pekarik Whitney Watriss FOREWORD This study of Genome: The Secret of How Life Works focuses on visitors’ perceptions of the exhibition. It draws attention to interactive aspects of the exhibition as well as to underlying conceptual dimensions: basic genome science and applied genome science. To produce information on the success of the exhibition, it captures responses to key factors in the exhibit, such as visitor satisfaction with respect to interactive activities and visitor satisfaction concerning physical attributes and explores the relationships among these factors through correlational analysis. The study was designed and administered by Ioana Munteanu, who analyzed the data and wrote this report; several colleagues from the Office of Policy and Analysis offered helpful advice on various parts of the study. Carole M. P. Neves Director Office of Policy and Analysis PART I. BACKGROUND Fifty years ago, Nobel Prize winners Francis Crick and James Watson first visualized the three-dimensional molecular structure of deoxyribonucleic acid’s (DNA) double helix. Since they accomplished that extraordinary feat, scientists have made remarkable progress in the field of modern genomics, including sequencing the human genome.1 The traveling exhibition, Genome: The Secret of How Life Works, sponsored by Pfizer and produced by Clear Channel Entertainment-Exhibition, opened for display in a 5,000- square foot gallery in the Smithsonian Institution’s Arts and Industries Building (A&I Building) on June 6, 2003. -

The Economic Impact and Functional Applications of Human Genetics and Genomics
The Economic Impact and Functional Applications of Human Genetics and Genomics Commissioned by the American Society of Human Genetics Produced by TEConomy Partners, LLC. Report Authors: Simon Tripp and Martin Grueber May 2021 TEConomy Partners, LLC (TEConomy) endeavors at all times to produce work of the highest quality, consistent with our contract commitments. However, because of the research and/or experimental nature of this work, the client undertakes the sole responsibility for the consequence of any use or misuse of, or inability to use, any information or result obtained from TEConomy, and TEConomy, its partners, or employees have no legal liability for the accuracy, adequacy, or efficacy thereof. Acknowledgements ASHG and the project authors wish to thank the following organizations for their generous support of this study. Invitae Corporation, San Francisco, CA Regeneron Pharmaceuticals, Inc., Tarrytown, NY The project authors express their sincere appreciation to the following indi- viduals who provided their advice and input to this project. ASHG Government and Public Advocacy Committee Lynn B. Jorde, PhD ASHG Government and Public Advocacy Committee (GPAC) Chair, President (2011) Professor and Chair of Human Genetics George and Dolores Eccles Institute of Human Genetics University of Utah School of Medicine Katrina Goddard, PhD ASHG GPAC Incoming Chair, Board of Directors (2018-2020) Distinguished Investigator, Associate Director, Science Programs Kaiser Permanente Northwest Melinda Aldrich, PhD, MPH Associate Professor, Department of Medicine, Division of Genetic Medicine Vanderbilt University Medical Center Wendy Chung, MD, PhD Professor of Pediatrics in Medicine and Director, Clinical Cancer Genetics Columbia University Mira Irons, MD Chief Health and Science Officer American Medical Association Peng Jin, PhD Professor and Chair, Department of Human Genetics Emory University Allison McCague, PhD Science Policy Analyst, Policy and Program Analysis Branch National Human Genome Research Institute Rebecca Meyer-Schuman, MS Human Genetics Ph.D. -

Genomic Sequencing of SARS-Cov-2: a Guide to Implementation for Maximum Impact on Public Health
Genomic sequencing of SARS-CoV-2 A guide to implementation for maximum impact on public health 8 January 2021 Genomic sequencing of SARS-CoV-2 A guide to implementation for maximum impact on public health 8 January 2021 Genomic sequencing of SARS-CoV-2: a guide to implementation for maximum impact on public health ISBN 978-92-4-001844-0 (electronic version) ISBN 978-92-4-001845-7 (print version) © World Health Organization 2021 Some rights reserved. This work is available under the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 IGO licence (CC BY-NC-SA 3.0 IGO; https://creativecommons.org/licenses/by-nc-sa/3.0/igo). Under the terms of this licence, you may copy, redistribute and adapt the work for non-commercial purposes, provided the work is appropriately cited, as indicated below. In any use of this work, there should be no suggestion that WHO endorses any specific organization, products or services. The use of the WHO logo is not permitted. If you adapt the work, then you must license your work under the same or equivalent Creative Commons licence. If you create a translation of this work, you should add the following disclaimer along with the suggested citation: “This translation was not created by the World Health Organization (WHO). WHO is not responsible for the content or accuracy of this translation. The original English edition shall be the binding and authentic edition”. Any mediation relating to disputes arising under the licence shall be conducted in accordance with the mediation rules of the World Intellectual Property Organization (http://www.wipo.int/amc/en/mediation/rules/). -

The Yale Center for Genome Analysis
The Yale Center for Genome Analysis A Core Research Facility at Yale’s West Campus Today, the Yale Center for Genome Analysis produces the equivalent of more than 3,000 complete human genomes a month, yielding an astonishing volume of information that drives research not only in human biology and medicine, but in every area of the life sciences. The Yale Center for Genome Analysis The first map of the human genome, announced in 2003 by Craig Venter and Francis Collins, was an astonishing accomplishment, requiring more than a decade of research, a $3 billion investment, and the work of 900 DNA sequencing machines in laboratories around the world. Today, the Yale Center for Genome Analysis produces the equivalent of more than 3,000 complete human genomes a month, yielding a tremendous volume of information that drives research not only in human biology and medicine, but in every area of the life sciences. This is indeed the age of genomics. The marriage of high-throughput screen- ing technologies and bioinformatics has created a powerful and expanding paradigm for discovery. Breakthrough technologies are enabling scientists to discern life’s patterns—as well as its most minute details—from a growing trove of genomic data, while powerful computational techniques open the resulting data to exacting analysis. The Yale Center for Genome Analysis, a state-of-the-art DNA sequencing facility located at Yale’s West Campus, is one of a small handful of academic laboratories in the nation equipped to support this kind of research at the cutting edge. O≠ering specialized services to sequence the genes and genomes of humans, animals, plants, and microbes, the Center supports wide-ranging inquiries into genetic functions and their e≠ects. -

Written Evidence Submitted by the MRC Human Genetics Unit at The
Written evidence submitted by the MRC Human Genetics Unit at the University of Edinburgh and the MRC Protein Phosphorylation and Ubiquitylation Unit at the University of Dundee (USC0001) We write in response to the UK Parliament Scottish Affairs Committee’s Call for Evidence in relation to Universities and Scotland. Our evidence pertains to “How Scottish university research fits in with UK university research”. We write as the directors of two UKRI Medical Research Council (MRC) Units based in Scottish Universities; the MRC Human Genetics Unit at the University of Edinburgh (https://www.ed.ac.uk/mrc-human-genetics-unit) and the MRC Protein Phosphorylation and Ubiquitylation Unit at the University of Dundee (https://www.ppu.mrc.ac.uk). As part of UKRI, the MRC invests 40% of its annual budget on an intramural portfolio of institutes, units and centres. MRC Units are long term investments that tackle important, often high risk, research questions that cannot be addressed adequately through short-term response-mode grant funding. MRC Units develop new methods and research facilities, foster interdisciplinarity, and spearhead training of the next generation of research leaders. Most MRC Units are embedded within universities (MRC University Units) and, because of the scale and sustainability of their research funding - reviewed and renewed every 5 years, they have a major impact on the research landscape of the universities that they are embedded in, including in Scotland. The scale and quality of research funded in MRC Units greatly enhances academic leadership within the host university as well as the Research Excellence Grant (REG) income that the host university receives from the Scottish Funding Council. -

Lectures and Seminars, Michaelmas Term 2014
WEDNESDay 8 octobEr 2014 • SUPPLEMENt (1) to No 5071 • VoL 145 Gazette Supplement Lectures and Seminars, Michaelmas term 2014 Romanes Lecture 26 Pharmacology, anatomical reuters Institute for the Study of Neuropharmacology and Drug Journalism Discovery Seminars Latin american centre Charles Simonyi Lecture 26 Physiology, anatomy and Genetics Foundation for Law, Justice and Society Population Health Maison Française Humanities 26 Psychiatry oxford Martin School Museum of Natural History TORCH | The Oxford Research Centre in Social Sciences 35 the Humanities Population ageing Classics anthropology and Museum Ethnography Ian ramsey centre English Language and Literature Archaeology Rhodes House English /History/History of Art/Theology/ Education McDonald centre for theology, Ethics and Public Life Music International Development (Queen History Elizabeth House) Colleges, Halls and Societies 47 History of Art Internet Institute Linguistics, Philology and Phonetics Law All Souls Medieval and Modern Languages Politics and International relations Green templeton Music Social Policy and Intervention Keble Oriental Studies Socio-legal Studies Kellogg Philosophy Sociology Mansfield Theology and Religion Nuffield Department for Continuing St Antony’s Education 40 Mathematical, Physical and St Catherine’s Life Sciences 32 Kellogg college centre for creative St Edmund Hall Chemistry Writing St Hilda’s Earth Sciences St John’s Institutes, Centres and Engineering Science Somerville Museums 41 e-Research Centre University college Materials ashmolean Museum -

Decoding Non-Coding DNA: Trash Or Treasure?
GENERAL ARTICLE Decoding Non-Coding DNA: Trash or Treasure? Namrata Iyer Non-coding DNA, once thought of as ‘junk’, represents a very large portion of an organism’s genome. However, recent research has brought to light many functional elements present within non-coding DNA sequences and unravelled a fascinat- ing array of functions performed by these elements. These findings have highlighted the nature of the evolutionary forces that led to the accumulation and retention of non-coding Namrata Iyer is a PhD DNA. In this article, the various elements present within non- student in the Department of Microbiology and Cell coding DNA, their functional relevance to the cell and the Biology, Indian Institute of changing perspective of the scientific community towards this Science, Bangalore. Her so-called ‘junk’ DNA have been described. research interest is the molecular basis of host– Since the dawn of time, man has always been plagued by the pathogen interactions in question of the origin of life. What are the forces that govern the human diseases. course of evolution? What are the elements that separate man from other forms of life? The discovery of DNA (deoxyribo- nucleic acid) as the genetic material in 1944 opened up new avenues to answer these questions. Surprisingly, the language of DNA comprises of only 4 letters, i.e., A,T,G,C which when read in groups of three (triplets) encode the information for the synthe- sis of proteins (by a process known as translation) which are the work-horses of a cell. Before translation can begin, the informa- tion on DNA is first copied into an intermediate known as mRNA (by a process known as transcription).