Ontogenetic Strategies in Insect Herbivores and Their Impact on Tri
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Phylogeny and Evolution of Lepidoptera
EN62CH15-Mitter ARI 5 November 2016 12:1 I Review in Advance first posted online V E W E on November 16, 2016. (Changes may R S still occur before final publication online and in print.) I E N C N A D V A Phylogeny and Evolution of Lepidoptera Charles Mitter,1,∗ Donald R. Davis,2 and Michael P. Cummings3 1Department of Entomology, University of Maryland, College Park, Maryland 20742; email: [email protected] 2Department of Entomology, National Museum of Natural History, Smithsonian Institution, Washington, DC 20560 3Laboratory of Molecular Evolution, Center for Bioinformatics and Computational Biology, University of Maryland, College Park, Maryland 20742 Annu. Rev. Entomol. 2017. 62:265–83 Keywords Annu. Rev. Entomol. 2017.62. Downloaded from www.annualreviews.org The Annual Review of Entomology is online at Hexapoda, insect, systematics, classification, butterfly, moth, molecular ento.annualreviews.org systematics This article’s doi: Access provided by University of Maryland - College Park on 11/20/16. For personal use only. 10.1146/annurev-ento-031616-035125 Abstract Copyright c 2017 by Annual Reviews. Until recently, deep-level phylogeny in Lepidoptera, the largest single ra- All rights reserved diation of plant-feeding insects, was very poorly understood. Over the past ∗ Corresponding author two decades, building on a preceding era of morphological cladistic stud- ies, molecular data have yielded robust initial estimates of relationships both within and among the ∼43 superfamilies, with unsolved problems now yield- ing to much larger data sets from high-throughput sequencing. Here we summarize progress on lepidopteran phylogeny since 1975, emphasizing the superfamily level, and discuss some resulting advances in our understanding of lepidopteran evolution. -

Bignoniaceae) in a Costa Rica Dry Forest (Lepidoptera: Pyralidae: Chrysauginae)
Vol. 11 No. 1-2 2000 SOUS et al: A New Chrysaugine Moth from Costa Rica 33 TROPICAL LEPIDOPTERA, 11(1): 33-39 (2003) CROMARCHA STROUDAGNESIA, A NEW CHRYSAUGINE SPECIES BORING IN SHOOTS OF TABEBUIA OCHRACEA (BIGNONIACEAE) IN A COSTA RICA DRY FOREST (LEPIDOPTERA: PYRALIDAE: CHRYSAUGINAE) M. ALMA SOLIS1, JON J. SULLIVAN23 and DANIEL H. JANZEN2 'Systematic Entomology Laboratory, PSI, ARS, USDA, National Museum of Natural History, MRC 168, Washington, DC 20560-0168 asolis @ sel.barc.usda.gov 2Dept. of Biology, University of Pennsylvania, Philadelphia, Pennsylvania 19104-6018, USA [email protected] ABSTRACT.- Cromarcha stroudagnesia Solis, n. sp., from the dry-forested Pacific coastal lowlands of northwestern to central Costa Rica and from the state of Jalisco in Mexico is described. Information on the biology and immature stages is also presented. The larvae bore inside the shoots of new rainy season growth of Tabebuia ochracea (Bignoniaceae) saplings. T. ochracea is known as corteza amarilla and is a tropical timber tree in Costa Rica. KEYWORDS: biology, Braconidae, Central America, chaetotaxy, Chalcidoidea, Clydonopteron, Cromarcha stroudagnesia n.sp., Cryploses, Guanacaste, Hymenoptera, immatures, larva, Mesoamerica, Mexico, Neotropical, parasitoids, pupa, Santa Rosa, taxonomy. The Chrysauginae are a large subfamily with about 400 sclerotized setae as long as pilifer. Labial palpus porrect, sexually dimor- described species in the Western Hemisphere (Solis et al., 1995). phic, female labial palpus almost twice as long as that of male; male with The Neotropical chrysaugines have never been revised, but Cashatt second segment 5 times longer than third segment, third segment medially with many very short heavily sclerotized setae, vom Rath's organ 2 times (1968) revised the Nearctic species and included a few genera that smaller than that of female; female with second segment 2.5 times longer occur in the Neotropics. -

A New Species of Galleria Fabricius (Lepidoptera, Pyralidae) from Korea
ZooKeys 970: 51–61 (2020) A peer-reviewed open-access journal doi: 10.3897/zookeys.970.54960 RESEARCH ARTIclE https://zookeys.pensoft.net Launched to accelerate biodiversity research A new species of Galleria Fabricius (Lepidoptera, Pyralidae) from Korea based on molecular and morphological characters Seung Jin Roh1, Haechul Park1, Seong-Hyun Kim1, So-Yun Kim1, Yong-Su Choi1, Jeong-Hun Song1 1 Department of Agricultural Biology, National Institute of Agricultural Sciences, Wanju 55365, South Korea Corresponding author: Jeong-Hun Song ([email protected]) Academic editor: Colin Plant | Received 8 June 2020 | Accepted 4 August 2020 | Published 21 September 2020 http://zoobank.org/8069F755-8DF6-4AEB-A1D7-FD20096B4C5C Citation: Roh SJ, Park H, Kim S-H, Kim S-Y, Choi Y-S, Song J-H (2020) A new species of Galleria Fabricius (Lepidoptera, Pyralidae) from Korea based on molecular and morphological characters. ZooKeys 970: 51–61. https:// doi.org/10.3897/zookeys.970.54960 Abstract The greater wax moth, Galleria mellonella Linnaeus, is well known as a pest of honey bees and for the biodegradation of wax and polyethylene by their larvae. The genus Galleria has long been considered monotypic and found worldwide. A taxonomic study of the genus Galleria is presented based on morpho- logical and molecular characters (COI, CAD, wg). A new species (Galleria similis Roh & Song, sp. nov.) is recognized on the Korean peninsula. The new species is superficially similar to G. mellonella but they can be separated by the structures of hindwing venation and male genitalia. Habitus photographs and illustra- tions of diagnostic characters are provided. Keywords cryptic species, Galleriinae, new species, plastic eating moth, Pyraloidea, wax worms Copyright Seung Jin Roh et al. -

Annotated Check List of the Pyraloidea (Lepidoptera) of America North of Mexico
A peer-reviewed open-access journal ZooKeys 535: 1–1136Annotated (2015) check list of the Pyraloidea (Lepidoptera) of America North of Mexico 1 doi: 10.3897/zookeys.535.6086 CHECKLIST http://zookeys.pensoft.net Launched to accelerate biodiversity research Annotated check list of the Pyraloidea (Lepidoptera) of America North of Mexico Brian G. Scholtens1, M. Alma Solis2 1 BGS, Biology Department, College of Charleston, Charleston, SC, USA 29424 2 MAS, Systematic Ento- mology Laboratory, USDA, National Museum of Natural History, P.O. Box 37012, MRC 168, Washington, DC USA 20013-7012 Corresponding author: Brian G. Scholtens ([email protected]) Academic editor: Matthias Nuss | Received 16 June 2015 | Accepted 14 October 2015 | Published 13 November 2015 http://zoobank.org/CE652AF8-014F-41A4-99E7-8F8BF1C6B0A4 Citation: Scholtens BG, Solis AM (2015) Annotated check list of the Pyraloidea (Lepidoptera) of America North of Mexico. ZooKeys 535: 1–136. doi: 10.3897/zookeys.535.6086 Abstract An annotated check list of Pyraloidea of North America north of Mexico is presented, including 861 Crambidae and 681 Pyralidae with 1542 total species. It includes all new species described, tropical species with new records in the United States, and species introduced from Europe and Asia since 1983. The Notes section provides the seminal citations, data and/or commentary to all changes since 1983 for easy and future reference. In addition, this list proposes seven new generic combinations, the transfer of a phycitine species, Salebria nigricans (Hulst), to Epipaschiinae and its syn. n. with Pococera fuscolotella (Ragonot), and three new records for the United States. Purposefully, no new taxa are described here, but we found a gradual increase of 10% in the number of species described since 1983. -

Moths of the Kingston Study Area
Moths of the Kingston Study Area Last updated 30 July 2015 by Mike Burrell This checklist contains the 783 species known to have occurred within the Kingston Study. Major data sources include KFN bioblitzes, an earlier version created by Gary Ure (2013) and the Queen’s University Biological Station list by Kit Muma (2008). For information about contributing your sightings or to download the latest version of this checklist, please visit: http://kingstonfieldnaturalists.org/moths/moths.html Contents Superfamily: Tineoidea .................................................................................................................................................... 5 Family: Tineidae ........................................................................................................................................................... 5 Subfamily: Tineinae .................................................................................................................................................. 5 Family: Psychidae ......................................................................................................................................................... 5 Subfamily: Psychinae ................................................................................................................................................ 5 Superfamily: Gracillarioidea ............................................................................................................................................. 5 Family: Gracillariidae ................................................................................................................................................... -
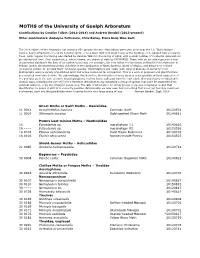
Moth Records
MOTHS of the University of Guelph Arboretum Contributions by Candice Talbot (2012-2014) and Andrew Bendall (2013-present) Other contributors: Andalyne Tofflemire, Chris Earley, Fiona Reid, Mike Kent The 2018 edition of the Arboretum list contains 851 species of moth. Most adults were seen at or near the J.C. Taylor Nature Centre, being attracted to the white building lights, or to a black light and sheet hung by the building, or to painted bait on nearby trees. Semi-regular monitoring was started by Candice Talbot in the spring of 2012, with a small number of incidental observations pre-dating that time. First record dates, where known, are shown at right as YYYYMMDD. Those with an asterisk represent a first documented sighting if the date of an earlier record was not available. We now follow the taxonomy outlined in Pohl, Patterson & Pelham (2016) Annotated taxonomic checklist of the Lepidoptera of North America, North of Mexico, and adopt their revised numbering system for all valid North American species. Identifications are made (with varying degrees of certainty) from photographs using a variety of published print and online resources for comparison. This is a work in progress and identifications are revisited from time to time. We acknowledge that definitive identification of some species is not possible without dissection of the genitalia or, in the case of some microlepidoptera, rearing larvae collected from the host plant. Our uncertainty is indicated in various ways, including the use of [t] for a tentative identification, by indicating a group of species that can't be separated from external features, or by identifying to genus only. -
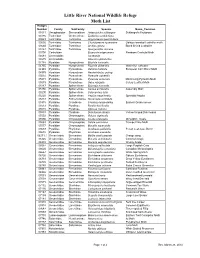
Little River NWR Moth List
Little River National Wildlife Refuge Moth List Hodges Number Family SubFamily Species Name_Common 01011 Oecophoridae Stenomatinae Antaeotricha schlaegeri Schlaeger's Fruitworm 03186 Tortricidae Olethreutinae Epiblema scudderiana 03623 Tortricidae Tortricinae Argyrotaenia quercifoliana 03635 Tortricidae Tortricinae Choristoneura rosaceana Oblique-banded Leafroller moth 03660 Tortricidae Tortricinae Archips grisea Black Shield Leafroller 03727 Tortricidae Tortricinae Sparganothis niveana 03790 Cochylidae Eugnosta erigeronana Fleabane Cochylid Moth 04681 Limacodidae Isa textula 04685 Limacodidae Adoneta spinuloides 04748 Pyralidae Nymphulinae Elophila icciusalis 04755 Pyralidae Nymphulinae Elophila obliteralis Waterlily Leafcutter 04949 Pyralidae Pyraustinae Ostrinia nubilalis European Corn Borer Moth 04979 Pyralidae Pyraustinae Neohelvibotys polingi 05034 Pyralidae Pyraustinae Pyrausta signatalis 05071 Pyralidae Pyraustinae Pyrausta acrionalis Mint-loving Pyrausta Moth 05079 Pyralidae Pyraustinae Udea rubigalis Celery Leaftier Moth 05147 Pyralidae Spilomelinae Epipagis huronalis 05150 Pyralidae Spilomelinae Samea ecclesialis Assembly Moth 05200 Pyralidae Spilomelinae Colomychus talis 05226 Pyralidae Spilomelinae Palpita magniferalis Splendid Palpita 05313 Pyralidae Schoenobiinae Donacaula sordidella 05378 Pyralidae Crambinae Crambus laqueatellus Eastern Grass-veneer 05512 Pyralidae Pyralinae Pyralis disciferalis 05518 Pyralidae Pyralinae Aglossa cuprina 05533 Pyralidae Pyralinae Dolichomia olinalis Yellow-fringed Dolichomia 05552 Pyralidae -

An Annotated List of the Lepidoptera of Honduras
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Center for Systematic Entomology, Gainesville, Insecta Mundi Florida 2-29-2012 An annotated list of the Lepidoptera of Honduras Jacqueline Y. Miller University of Florida, [email protected] Deborah L. Matthews University of Florida, [email protected] Andrew D. Warren University of Florida, [email protected] M. Alma Solis Systematic Entomology Laboratory, PSI, Agriculture Research Service, USDA, [email protected] Donald J. Harvey Smithsonian Institution, Washington, D.C., [email protected] See next page for additional authors Follow this and additional works at: https://digitalcommons.unl.edu/insectamundi Part of the Entomology Commons Miller, Jacqueline Y.; Matthews, Deborah L.; Warren, Andrew D.; Solis, M. Alma; Harvey, Donald J.; Gentili- Poole, Patricia; Lehman, Robert; Emmel, Thomas C.; and Covell, Charles V., "An annotated list of the Lepidoptera of Honduras" (2012). Insecta Mundi. 725. https://digitalcommons.unl.edu/insectamundi/725 This Article is brought to you for free and open access by the Center for Systematic Entomology, Gainesville, Florida at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Insecta Mundi by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. Authors Jacqueline Y. Miller, Deborah L. Matthews, Andrew D. Warren, M. Alma Solis, Donald J. Harvey, Patricia Gentili-Poole, Robert Lehman, Thomas C. Emmel, and Charles V. Covell This article is available at DigitalCommons@University of Nebraska - Lincoln: https://digitalcommons.unl.edu/ insectamundi/725 INSECTA A Journal of World Insect Systematics MUNDI 0205 An annotated list of the Lepidoptera of Honduras Jacqueline Y. Miller, Deborah L. -

Order Family Subfamily Genus Species Subspecies Author Year Series Region Units Lepidoptera Crambidae Acentropinae Acentria Ephe
Order Family Subfamily Genus species subspecies author year series region units Lepidoptera Crambidae Acentropinae Acentria ephemerella (Denis & Schiffermüller) 1C, 1D Nearctic, Palearctic trays Lepidoptera Crambidae Acentropinae Anydraula glycerialis (Walker) 1D Australasian trays Lepidoptera Crambidae Acentropinae Argyractis berthalis (Schaus) 1C Neotropical trays Lepidoptera Crambidae Acentropinae Argyractis dodalis Schaus 1B Neotropical trays Lepidoptera Crambidae Acentropinae Argyractis elphegalis (Schaus) 1B Neotropical trays Lepidoptera Crambidae Acentropinae Argyractis flavalis (Warren) 1B Neotropical trays Lepidoptera Crambidae Acentropinae Argyractis iasusalis (Walker) 1D Neotropical trays Lepidoptera Crambidae Acentropinae Argyractis paulalis (Schaus) 1D Neotropical trays Lepidoptera Crambidae Acentropinae Argyractis sp. 1C, 1D Neotropical trays Lepidoptera Crambidae Acentropinae Argyractis tetropalis Hampson 1D African trays Lepidoptera Crambidae Acentropinae Argyractis triopalis Hampson 1D African trays Lepidoptera Crambidae Acentropinae Argyractoides catenalis (Guenée 1D Neotropical trays Lepidoptera Crambidae Acentropinae Argyractoides chalcistis (Dognin) 1D Neotropical trays Lepidoptera Crambidae Acentropinae Argyractoides gontranalis (Schaus) 1D Neotropical trays Lepidoptera Crambidae Acentropinae Aulacodes acroperalis Hampson 1D Australasian trays Lepidoptera Crambidae Acentropinae Aulacodes adiantealis (Walker) 1D Neotropical trays Lepidoptera Crambidae Acentropinae Aulacodes aechmialis Guenée 1D Neotropical trays Lepidoptera -

2017-2018 Annual Report, Most Likely It Would Be “Transitions.”
FROM THE DIRECTOR If I had to select a title for this 2017-2018 Annual Report, most likely it would be “Transitions.” From my perspective as Director, I feel we witnessed an unprecedented number of important changes to our Museum personnel, programs and philosophies this year. Florida Museum For example, in January 2018 we transitioned into our second century as Florida’s state museum of natural of Natural History history. We celebrated the final chapter of our 100th anniversary year (2017) with the centennial exhibition - Rare, Beautiful and Fascinating: 100 Years @FloridaMuseum. The exhibit highlighted the enormous accomplishments of the Museum’s first century, which resulted in more than 40 million specimens and 2017-2018 Annual Report objects under our curatorial care. Certainly we’ll never stop collecting, but our research and collections emphasis is also transitioning to digitization and data sharing to address important scientific questions and challenging environmental issues. The transition from pure collection growth and curation to data mining for the benefit of science and society represents a subtle shift in our traditional museum philosophy. Always proud of our collections and exhibitions, we are increasingly concerned with creating a positive impact on the diverse communities we serve, from scientists and university students, to K-12 youth and the general public. Like so many of our peer institutions, this means we need to “turn the Museum inside-out,” better understand our audiences, appreciate their needs and concerns, and find ways to touch their lives. Our investments in digitization, social media, Museum pop-ups, and school programs all reflect this commitment to audience impact. -

Phylogeny, Character Evolution and Species Diversity in Crambinae, Heliothelinae and Scopariinae
Phylogeny, character evolution and species diversity in Crambinae, Heliothelinae and Scopariinae DISSERTATION zur Erlangung des akademischen Grades DOCTOR RERUM NATURALIUM (Dr. rer. nat.) vorgelegt dem Bereich Mathematik und Naturwissenschaften der Technischen Universit¨at Dresden von M. Sc. Th´eo Antonin Baptiste L´eger geboren am 02.01.1989 in Lausanne, Schweiz eingereicht am 10. Januar 2020 Die Dissertation wurde in der Zeit von 09/2014 bis 12/2019 an den Senckenberg Naturhistorischen Sammlungen Dresden angefertigt. ii Charles Darwin, letter to T. H. Huxley, 26 September 1857 Darwin Correspondence Project, “Letter no. 2143”, https://www.darwinproject.ac.uk/letter/DCP-LETT-2143.xml 1. Gutachter 2. Gutachter Prof. Dr. Christoph Neinhuis Prof. Dr. Niklas Wahlberg Lehrstuhl f¨ur Botanik, Systematic Biology Group Fakult¨at Mathematik und Faculty of Science Naturwissenschaften Lund University Technische Universit¨at Dresden S¨olvegatan 37, Lund Dresden, Deutschland Schweden Declaration Erkl¨arung gem¨aß § 5.1.5 der Promotionsordnung Hiermit versichere ich, dass ich die vorliegende Arbeit ohne unzul¨assigeHilfe Dritter und ohne Benutzung anderer als der angegebenen Hilfsmittel angefertigt habe; die aus fremden Quellen direkt oder indirektubernommenen ¨ Gedanken sind als solche kenntlich gemacht. Die Arbeit wurde bisher weder im Inland noch im Ausland in gleicher oder ¨ahnlicher Form einer anderen Pr¨ufungsbeh¨orde vorgelegt. Berlin, 14. Januar 2020 Th´eo L´eger iii iv Acknowledgements This work would not have been possible without the help and support from various people. I want to express my sincere gratitude to Matthias Nuss and Bernard Landry for introducing me to the fabulous group that represent Pyraloidea and to the thrilling field of research that is systematics. -

Some Iowa Records of Lepidoptera
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by University of Northern Iowa Proceedings of the Iowa Academy of Science Volume 27 Annual Issue Article 59 1920 Some Iowa Records of Lepidoptera A. W. Lindsey The Barnes' Museum Copyright ©1920 Iowa Academy of Science, Inc. Follow this and additional works at: https://scholarworks.uni.edu/pias Recommended Citation Lindsey, A. W. (1920) "Some Iowa Records of Lepidoptera," Proceedings of the Iowa Academy of Science, 27(1), 319-335. Available at: https://scholarworks.uni.edu/pias/vol27/iss1/59 This Research is brought to you for free and open access by the Iowa Academy of Science at UNI ScholarWorks. It has been accepted for inclusion in Proceedings of the Iowa Academy of Science by an authorized editor of UNI ScholarWorks. For more information, please contact [email protected]. Lindsey: Some Iowa Records of Lepidoptera SOME row j\ RECORDS OF LEPIDOPTERA AW. LINDSEY My first contribution to the study of the Lepidoptera of Iowa appeared in the Proceedings of the Academy for 1914 in the form of a list of butterflies, exclusive of skippers, taken in Wood bury county (Proc. Ia. Acad. Sci. xxi, 1914, 341-346). Later I was able to complete the identification of the skippers in my possession and to examine a number of collections in various parts of the state, thus increasing my list to such an extent that in 1917 I published a second list, embracing the entire state and including all of the Diurnals (Ent. News xxviii, 1917, 347-353).