Bivalves for the Remediation of Prawn Farm Effluent: Identification of Some Potentially Useful Species in Southern Queensland’
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Oyster Aquaculture Production Systems
UNIVERSITY OF MARYLAND EXTENSION //////////// extension.umd.edu ///////////////////////////////////////////////////////////////////////////////////////////////////////////// EM- 7 | November 2019 //////////// Oyster Aquaculture Production Systems Growers have cultured oysters for thousands of years and used Many Factors will Influence the System You many methods to raise them. The methods are based on the Choose to Raise Oysters technology, materials, species, environmental conditions, labor and other parameters available in the area where oysters are Before making a choice, investigate as many different systems raised. as you can, keeping in mind the location of your farm, Some growers will let you visit their operation or you may be “You don’ t make money familiar with local farms already in production. Oyster farmers raising oysters; you make are often comfortable showing methods and equipment they money by selling them. ” use because they know that expanding production benefits the entire industry. As a longtime grower once said, “What makes While there are many methods used to raise oysters, not all are my company profitable doesn’t show up in photos – it’s the profitable for Maryland growers. Profitable operation is the most management of the business that makes it successful.” important part of a successful aquaculture business. As a grower, you should make decisions based on sound business practices. Information, advice and training are available through Your target markets will also determine the best methods of University of Maryland Extension specialists. Workshops and oyster production. Oysters produced specifically for the seminars are held throughout the year on such topics as half-shell or raw bar trade are often cultchless. They are production systems, business management and seafood produced by setting larvae on small shell pieces rather than technology to provide the training necessary for industry growth multiple animals on a single shell as is done for bottom leases. -

Marine Bivalve Molluscs
Marine Bivalve Molluscs Marine Bivalve Molluscs Second Edition Elizabeth Gosling This edition first published 2015 © 2015 by John Wiley & Sons, Ltd First edition published 2003 © Fishing News Books, a division of Blackwell Publishing Registered Office John Wiley & Sons, Ltd, The Atrium, Southern Gate, Chichester, West Sussex, PO19 8SQ, UK Editorial Offices 9600 Garsington Road, Oxford, OX4 2DQ, UK The Atrium, Southern Gate, Chichester, West Sussex, PO19 8SQ, UK 111 River Street, Hoboken, NJ 07030‐5774, USA For details of our global editorial offices, for customer services and for information about how to apply for permission to reuse the copyright material in this book please see our website at www.wiley.com/wiley‐blackwell. The right of the author to be identified as the author of this work has been asserted in accordance with the UK Copyright, Designs and Patents Act 1988. All rights reserved. No part of this publication may be reproduced, stored in a retrieval system, or transmitted, in any form or by any means, electronic, mechanical, photocopying, recording or otherwise, except as permitted by the UK Copyright, Designs and Patents Act 1988, without the prior permission of the publisher. Designations used by companies to distinguish their products are often claimed as trademarks. All brand names and product names used in this book are trade names, service marks, trademarks or registered trademarks of their respective owners. The publisher is not associated with any product or vendor mentioned in this book. Limit of Liability/Disclaimer of Warranty: While the publisher and author(s) have used their best efforts in preparing this book, they make no representations or warranties with respect to the accuracy or completeness of the contents of this book and specifically disclaim any implied warranties of merchantability or fitness for a particular purpose. -

Spawning and Rearing Bivalve Molluscs—Spawning1 R
FA174 Teach Aquaculture Curriculum: Spawning and Rearing Bivalve Molluscs—Spawning1 R. Leroy Creswell, Cortney L. Ohs, Craig S. Kasper, Elisa J. Livengood, Amber L. Garr, Brian E. Myers, Carlos V. Martinez, and Frank A. Chapman2 This is Activity 12 in a series of 25 in the Teach Aquaculture Grade Level Curriculum.The introduction to this series is available at 9–12 http://edis.ifas.ufl.edu/FA177. Abstract Subject Area Biology, Aquaculture In this activity students will learn methods for spawning bivalve molluscs like clams or oysters using temperature manipulation. Students will use an ocular micrometer to Time measure the diameter of bivalve eggs and the length of Preparation: Spawning—indeterminant; daily exchange 10 bivalve larvae. The following lesson plan will have students minutes monitor the density of larvae in their culture system and Activity: Spawning—indeterminant; daily exchange 20 understand production protocols used in bivalve hatcheries. minutes Clean-up: 10 minutes Objectives Students will be able to: Student Performance Standards (Sunshine State Standards) 1. Describe the reproductive biology and spawning of bivalve molluscs. 06.03 Illustrate correct terminologies for animal species and conditions (e.g., sex, age, etc.) within those species 2. Apply techniques used for spawning molluscs. (LA.910.1.6.1, 2, 3, 4, 5; SC.912.L.14. 19, 31, 33). 3. Describe the conditions used in hatcheries for commer- cial production of bivalve molluscs. 1. This document is FA174, one of a series of the School of Forest Resources and Conservation, Program in Fisheries and Aquatic Sciences, UF/IFAS Extension. Original publication date May 2010. -

Invasive Seaweed Enhances Recruitment of a Native Bivalve: Roles of Refuge from Predation and the Habitat Choice of Recruits
MARINE ECOLOGY PROGRESS SERIES Vol. 318: 177–185, 2006 Published August 3 Mar Ecol Prog Ser Invasive seaweed enhances recruitment of a native bivalve: roles of refuge from predation and the habitat choice of recruits Paul E. Gribben1,*, Jeffrey T. Wright2 1Centre for Marine Biofouling and Bio-Innovation, University of New South Wales, New South Wales 2052, Australia 2Institute for Conservation Biology and School of Biological Sciences, University of Wollongong, New South Wales 2522, Australia ABSTRACT: Invasive species may have a range of negative effects on native species in the region invaded. The invasive green alga Caulerpa taxifolia has invaded several temperate regions world- wide and now occurs in 9 estuaries in temperate eastern Australia. Despite the threat posed by C. taxifolia, virtually nothing is known of its effects on native estuarine infauna. In the present study, we investigated the distribution and abundance, habitat choice and predation of recruits (post-set juveniles) of the native Sydney cockle Anadara trapezia at 2 sites invaded by C. taxifolia in Lake Conjola, New South Wales, Australia. Recruitment of A. trapezia was significantly higher in C. taxi- folia (both with sparse [30%] and with dense [100%] cover) than in Zostera capricorni and bare sed- iment. Up to 680 recruits m–2 were observed in C. taxifolia, with the highest recruit densities occur- ring at intermediate C. taxifolia densities. However, in habitat choice experiments, recruits showed no preference for C. taxifolia over the seagrasses Z. capricorni and Halophila ovalis, but a strong pref- erence for adult A. trapezia over all macrophytes when A. trapezia were included as treatments in experiments. -
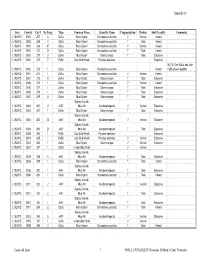
Copy of Appendix 5 Catalogue
Appendix 5.7 Area Context Cat # No.Frags Type Common Name Scientific Name Fragmentation Portion Shell Locality Comments L102W/D 3992 267 6 SaCu Rock Oyster Saccostrea cucullata V Ventral Varied L102W/D 3992 268 4 SaCu Rock Oyster Saccostrea cucullata T Total Varied L102W/D 3992 269 47 SaCu Rock Oyster Saccostrea cucullata V Ventral Varied L102W/D 3992 270 27 SaCu Rock Oyster Saccostrea cucullata T Total Varied L102W/D 3992 271 3 OsAn Mud Oyster Ostrea angasi T Total Estuarine L102W/D 3992 272 7 PyEb Club Mud Whelk Pyrazus ebeninus Estuarine NOTE: One SaCu and One L102W/D 3992 273 1 SaCu Rock Oyster Saccostrea cucullata Varied PyEb joined together L102W/D 3961 274 1 SaCu Rock Oyster Saccostrea cucullata V Ventral Varied L102W/D 3961 275 6 OsAn Mud Oyster Ostrea angasi T Total Estuarine L102W/C 3986 276 1 SaCu Rock Oyster Saccostrea cucullata V Ventral Varied L102W/C 3456 277 1 OsAn Mud Oyster Ostrea angasi T Total Estuarine L102W/C 3553 278 1 OsAn Mud Oyster Ostrea angasi T Total Estuarine L102W/C 3557 279 2 OsAn Mud Oyster Ostrea angasi T Total Estuarine Sydney Cockle, L102W/C 3684 280 2 AnTr Mud Ark Anadara trapezia V Ventral Estuarine L102W/C 3605 281 1 OsAn Mud Oyster Ostrea angasi T Total Estuarine Sydney Cockle, L102W/C 3684 282 22 AnTr Mud Ark Anadara trapezia V Ventral Estuarine Sydney Cockle, L102W/C 3684 283 23 AnTr Mud Ark Anadara trapezia T Total Estuarine L102W/C 3684 284 1 PyEb Club Mud Whelk Pyrazus ebeninus Estuarine L102W/C 3514 285 1 PyEb Club Mud Whelk Pyrazus ebeninus V Ventral Estuarine L102W/C 3514 286 1 OsAn Mud -

E Urban Sanctuary Algae and Marine Invertebrates of Ricketts Point Marine Sanctuary
!e Urban Sanctuary Algae and Marine Invertebrates of Ricketts Point Marine Sanctuary Jessica Reeves & John Buckeridge Published by: Greypath Productions Marine Care Ricketts Point PO Box 7356, Beaumaris 3193 Copyright © 2012 Marine Care Ricketts Point !is work is copyright. Apart from any use permitted under the Copyright Act 1968, no part may be reproduced by any process without prior written permission of the publisher. Photographs remain copyright of the individual photographers listed. ISBN 978-0-9804483-5-1 Designed and typeset by Anthony Bright Edited by Alison Vaughan Printed by Hawker Brownlow Education Cheltenham, Victoria Cover photo: Rocky reef habitat at Ricketts Point Marine Sanctuary, David Reinhard Contents Introduction v Visiting the Sanctuary vii How to use this book viii Warning viii Habitat ix Depth x Distribution x Abundance xi Reference xi A note on nomenclature xii Acknowledgements xii Species descriptions 1 Algal key 116 Marine invertebrate key 116 Glossary 118 Further reading 120 Index 122 iii Figure 1: Ricketts Point Marine Sanctuary. !e intertidal zone rocky shore platform dominated by the brown alga Hormosira banksii. Photograph: John Buckeridge. iv Introduction Most Australians live near the sea – it is part of our national psyche. We exercise in it, explore it, relax by it, "sh in it – some even paint it – but most of us simply enjoy its changing modes and its fascinating beauty. Ricketts Point Marine Sanctuary comprises 115 hectares of protected marine environment, located o# Beaumaris in Melbourne’s southeast ("gs 1–2). !e sanctuary includes the coastal waters from Table Rock Point to Quiet Corner, from the high tide mark to approximately 400 metres o#shore. -

Ripiro Beach
http://researchcommons.waikato.ac.nz/ Research Commons at the University of Waikato Copyright Statement: The digital copy of this thesis is protected by the Copyright Act 1994 (New Zealand). The thesis may be consulted by you, provided you comply with the provisions of the Act and the following conditions of use: Any use you make of these documents or images must be for research or private study purposes only, and you may not make them available to any other person. Authors control the copyright of their thesis. You will recognise the author’s right to be identified as the author of the thesis, and due acknowledgement will be made to the author where appropriate. You will obtain the author’s permission before publishing any material from the thesis. The modification of toheroa habitat by streams on Ripiro Beach A thesis submitted in partial fulfilment of the requirements for the degree of Master of Science (Research) in Environmental Science at The University of Waikato by JANE COPE 2018 ―We leave something of ourselves behind when we leave a place, we stay there, even though we go away. And there are things in us that we can find again only by going back there‖ – Pascal Mercier, Night train to London i Abstract Habitat modification and loss are key factors driving the global extinction and displacement of species. The scale and consequences of habitat loss are relatively well understood in terrestrial environments, but in marine ecosystems, and particularly soft sediment ecosystems, this is not the case. The characteristics which determine the suitability of soft sediment habitats are often subtle, due to the apparent homogeneity of sandy environments. -

New Report and Taxonomic Comparison of Anadara and Tegillarca Species of Arcidae (Bivalvia: Arcoidea) from Southern Coast of India
International Journal of Science and Research (IJSR) ISSN (Online): 2319-7064 Index Copernicus Value (2013): 6.14 | Impact Factor (2013): 4.438 New Report and Taxonomic Comparison of Anadara and Tegillarca Species of Arcidae (Bivalvia: Arcoidea) from Southern Coast of India Souji.S1, Tresa Radhakrishnan2 Department of Aquatic Biology and Fisheries, University of Kerala, Thiruvananthapuram-695 581, Kariyavattom, Kerala, India Abstract: Arcacea family is economically important group of animals. Most of the species in this family are misplaced into invalid subgenera and Indian arcids are wanted a revision in systematic positon. In the case of Arcidae family; all of the species are treated under Anadara as main genera, however, some authors considered that the Tegillarca genus is only a sub genus of Arcidae family. Anadara is the commercially important genus of bivalves of Arcidae family. These two genera are confused by many taxonomists and some considered that the morphometric changes of Tegillarca are only the habitual adaptation. But the collected samples from the same habitat from the southern part of India is clearly demarked the distinction between Anadara species and Tegillarca species. In this paper the differences between these two genera are illustrated with the help of specimens from the same habitat and with the help of taxonomic literature of these genera. Species level classification was done based on the morphometric characters like peculiarities of (i) periostracum, (ii) cardinal area, (iii) umbo, (iv) adductor muscle scar and (v) pallial line. The specimens were collected from Neendakara, Vizhinjam and Kovalam along with the south west coast and Thiruchendur in Tamil Nadu, south east coast of India. -

Aquaculture and Marine Protected Areas: Exploring Potential Opportunities and Synergies
Aquaculture and Marine Protected Areas: Exploring Potential Opportunities and Synergies To meet the Convention on Biological Diversity’s Aichi Target 11 on marine biodiversity protection, Aichi Target 6 on sustainable fisheries by 2020, as well as the Sustainable Development Goal (SDG) 2 on food security and SDG 14 on oceans, by 2030, there is an urgent need to reconcile nature conservation and sustainable development. It is also widely recognised that aquaculture significantly contributes to sustainable development in coastal communities and plays a vital role in ensuring food security, poverty alleviation, and economic resilience. In the framework of integrated management, the time has therefore come to identify the potential opportunities and synergies that can enable aquaculture and conservation to work together more effectively. CONTENT Understanding the various types of aquaculture and their potentialities ……………………………………… 3 The types of MPAs and matrix of interactions showing aquaculture & sustainability principles …… 7 Understanding aquaculture and MPA interactions …… 8 Towards MPAs and aquaculture compatibility and sustainability ……………………………………………10 Background In order to feed the world's growing human population, attention will need to increasingly focus on where the protein needs of the world will be supplied from. While capture fisheries have now reached a plateau of production, marine aquaculture of fish, shellfish and algae has been steadily increasing over the past decades and has become a valid option to make up the protein shortfall. However, one of the major constraints for the aquaculture production sector is the availability of, and access to space. In many coastal areas, competition with other marine activities is already high, mainly because the bulk of marine aquaculture is located close to the shore. -

Ostrea Angasi) Farming Industry in New South Wales
Paving the way for continued rapid development of the flat (angasi) oyster (Ostrea angasi) farming industry in New South Wales Mike Heasman1, Ben K. Diggles2, David Hurwood3, Peter Mather3, Igor Pirozzi1 and Symon Dworjanyn1 1NSW Fisheries Port Stephens Fisheries Centre Private Bag 1 Nelson Bay NSW 2315 2NIWA Australia Pty Ltd PO Box 359 Wilston Qld 4051 3School of Natural Resource Sciences Queensland University of Technology GPO Box 2434 Brisbane Qld 4001 Final Report to the Department of Transport & Regional Services Project No. NT002/0195 June 2004 NSW Fisheries Final Report Series No. 66 ISSN 1440-3544 Paving the way for continued rapid development of the flat (angasi) oyster (Ostrea angasi) farming industry in New South Wales Mike Heasman1, Ben K. Diggles2, David Hurwood3, Peter Mather3, Igor Pirozzi1 and Symon Dworjanyn1 1NSW Fisheries Port Stephens Fisheries Centre Private Bag 1 Nelson Bay NSW 2315 2NIWA Australia Pty Ltd PO Box 359 Wilston Qld 4051 3School of Natural Resource Sciences Queensland University of Technology GPO Box 2434 Brisbane Qld 4001 Final Report to the Department of Transport & Regional Services Project No. NT002/0195 June 2004 NSW Fisheries Final Report Series No.66 ISSN 1440-3544 Paving the way for continued rapid development of the flat (angasi) oyster (Ostrea angasi) farming in NSW June 2004 Authors: Michael P. Heasman, Ben K. Diggles, David Hurwood and Peter Mather, Igor Pirozzi and Symon Dworjanyn Published By: NSW Fisheries Postal Address: Private Bag 1, Nelson Bay NSW 2315 Internet: www.fisheries.nsw.gov.au NSW Fisheries and the Department of Transport & Regional Services This work is copyright. -

Atlas of the Freshwater Mussels (Unionidae)
1 Atlas of the Freshwater Mussels (Unionidae) (Class Bivalvia: Order Unionoida) Recorded at the Old Woman Creek National Estuarine Research Reserve & State Nature Preserve, Ohio and surrounding watersheds by Robert A. Krebs Department of Biological, Geological and Environmental Sciences Cleveland State University Cleveland, Ohio, USA 44115 September 2015 (Revised from 2009) 2 Atlas of the Freshwater Mussels (Unionidae) (Class Bivalvia: Order Unionoida) Recorded at the Old Woman Creek National Estuarine Research Reserve & State Nature Preserve, Ohio, and surrounding watersheds Acknowledgements I thank Dr. David Klarer for providing the stimulus for this project and Kristin Arend for a thorough review of the present revision. The Old Woman Creek National Estuarine Research Reserve provided housing and some equipment for local surveys while research support was provided by a Research Experiences for Undergraduates award from NSF (DBI 0243878) to B. Michael Walton, by an NOAA fellowship (NA07NOS4200018), and by an EFFRD award from Cleveland State University. Numerous students were instrumental in different aspects of the surveys: Mark Lyons, Trevor Prescott, Erin Steiner, Cal Borden, Louie Rundo, and John Hook. Specimens were collected under Ohio Scientific Collecting Permits 194 (2006), 141 (2007), and 11-101 (2008). The Old Woman Creek National Estuarine Research Reserve in Ohio is part of the National Estuarine Research Reserve System (NERRS), established by section 315 of the Coastal Zone Management Act, as amended. Additional information on these preserves and programs is available from the Estuarine Reserves Division, Office for Coastal Management, National Oceanic and Atmospheric Administration, U. S. Department of Commerce, 1305 East West Highway, Silver Spring, MD 20910. -

A Regional Shellfish Hatchery for the Wider Caribbean Assessing Its Feasibility and Sustainability
FAO ISSN 2070-6103 19 FISHERIES AND AQUACULTURE PROCEEDINGS FAO FISHERIES AND AQUACULTURE PROCEEDINGS 19 19 A regional shellfish hatchery for the Wider Caribbean Assessing its feasibility and sustainability A regional shellfish hatchery for the Wider Caribbean – Assessing its feasibility and sustainability A regional FAO Regional Technical Workshop A regional shellfish hatchery for 18–21 October 2010 Kingston, Jamaica the Wider Caribbean It is widely recognized that the development of aquaculture in Assessing its feasibility and sustainability the Wider Caribbean Region is inhibited, in part, by the lack of technical expertise, infrastructure, capital investment and human resources. Furthermore, seed supply for native species FAO Regional Technical Workshop relies, for the most part, on natural collection, subject to 18–21 October 2010 natural population abundance with wide yearly variations. This Kingston, Jamaica situation has led to the current trend of culturing more readily available exotic species, but with a potentially undesirable impact on the natural environment. The centralizing of resources available in the region into a shared facility has been recommended by several expert meetings over the past 20 years. The establishment of a regional hatchery facility, supporting sustainable aquaculture through the seed production of native molluscan species was discussed at the FAO workshop “Regional shellfish hatchery: A feasibility study” held in Kingston, Jamaica, in October 2010, by representatives of Caribbean Governments and experts in the field. Molluscan species are particularly targeted due to their culture potential in terms of known techniques, simple grow-out technology and low impact on surrounding environment. It is proposed that a regional molluscan hatchery would produce seed for sale and distribution to grow-out operations in the region as well as provide technical support for the research on new species.