Immunohistochemical Study of Intimal Microvessels in Coronary Atherosclerosis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Guide to Patient Support and Regional Cancer Services
Guide to Patient Support and Regional Cancer Services Information for cancer patients, carers and families in the ACT and surrounding region Accessibility The ACT Government is committed to making its information, services, events and venues, accessible to as many people as possible. • If you have difficulty reading a standard printed document and would like to receive this publication in an alternative format—such as large print or audio—please telephone 13 2281 or email [email protected]. • If English is not your first language and you require the translating and interpreting service—please telephone 131 450. • If you are deaf or hearing impaired and require the TTY typewriter service—please telephone (02) 13 3677, then ask for 13 2281. • Speak and listen users—phone 1300 555 727 then ask for 13 2281. • Internet Relay Users—connect to the NRS, then ask for 13 2281. © Australian Capital Territory, Canberra, October 2011 This work is copyright. Apart from any use as permitted under the Copyright Act 1968, no part may be reproduced by any process without written permission from the Territory Records Office, Community and Infrastructure Services, Territory and Municipal Services, ACT Government, GPO Box 158, Canberra City ACT 2601. Enquiries about this publication should be directed to ACT Government Health Directorate, Communications and Marketing Unit, GPO Box 825 Canberra City ACT 2601 or email: [email protected] www.health.act.gov.au | www.act.gov.au Enquiries: Canberra 13ACT1 or 132281 Publication No 11/0815 Contents Acknowledgments -
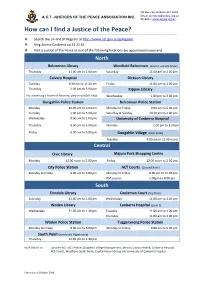
How Can I Find a JP
PO Box 766, Dickson ACT 2602 A.C.T. JUSTICES OF THE PEACE ASSOCIATION INC. Email: [email protected] Website: www.actjpa.org.au How can I find a Justice of the Peace? ❖ Search the on-line JP Register at http://www.act.gov.au/jpregister ❖ Ring Access Canberra on 13 22 81 ❖ Visit a Justice of the Peace at one of the following locations (no appointment necessary) North Belconnen Library Westfield Belconnen (level 3, outside Myer) Thursday 11.00 am to 1.00 pm Saturday 11.00 am to 1.00 pm Calvary Hospital Dickson Library Tuesday 9.30 am to 11.30 am Friday 11.00 am to 1.00 pm Thursday 1.00 pm to 3.00 pm Kippax Library For witnessing a Power of Attorney, please call 6201 6646. Wednesday 1.00 pm to 3.00 pm Gungahlin Police Station Belconnen Police Station Monday 10.00 am to 2.00 pm Monday to Friday 9.00 am to 5.00 pm Tuesday 1.00 pm to 5.00 pm Saturday & Sunday 10.30 am to 2.00 pm Wednesday 9.00 am to 1.00 pm University of Canberra Hospital Thursday 9.00 am to 5.00 pm Monday 1.00 pm to 3.00 pm Friday 9.00 am to 5.00 pm Gungahlin Village (near Coles) Tuesday 9.00 am to 12.00 noon Central Civic Library Majura Park Shopping Centre Monday 12.00 noon to 2.00 pm Friday 12.00 noon to 2.00 pm City Police Station ACT Courts (Ground floor) Monday to Friday 9.00 am to 5.00 pm Monday to Friday 9.30 am to 12.30 pm PM session 1.00pm to 4.00 pm . -

Federal Hansard Acronyms List Remember: Ctrl+F for Quick Searches
Federal Hansard Acronyms List Remember: Ctrl+F for quick searches A B C D E F G H I J K L M N O P Q R S T U V W X Y Z A 2.5G [the first packet overlays on 2G networks] 2G second generation [the first generation of digital cellular networks, as opposed to analog] 3G third generation [next generation of cellular networks] 3GPP 3G Partnership Project [global standards body to oversee 3G] 4D meat from dead, dying, diseased or disabled animals 4GL fourth-generation language [computers] A&C automation and control A&D admission and disposition; alcohol and drugs A&E accident and emergency A&RMC formerly Austin & Repatriation Medical Centre [now Austin Health] AA anti-aircraft; Alcoholics Anonymous; Athletics Australia AAA Agriculture Advancing Australia; Australian Automobile Association; Australian Archaeological Association; Australian Airports Association AAAA Aerial Agricultural Association of Australia AAAE Australian Association of Automotive Electricians AAAGP Australian Association of Academic General Practice AAALAC Association for the Assessment and Accreditation of Laboratory Animal Care International AAB Australian Associated Brewers AAC Aboriginal advisory committee; Australian Arabic Council; AARNet Advisory Committee AACAP ATSIC-Army Community Assistance Program AACC Aboriginal Affairs Coordinating Committee [WA]; Australian Association of Career Counsellors AACM Australian Association for Computational Mechanics AACS Australian Associations of Christian Schools [note: Associations—plural] AACV Australian Association of Cattle Veterinarians AAD Australian Antarctic Division [Department of the Environment and Heritage] AADCP ASEAN-Australia Development Cooperation Program [taking over AAECP] AADS advanced air defence simulator AADT average annual daily traffic AaE Australian air Express Pty Ltd AAEC Antarctic Animal Ethics Committee AAECP ASEAN-Australia Economic Cooperation Program [finishes in 2005] AAFCANS Army and Air Force Canteen Service [now known as Frontline Defence Services] AAGP Australian Association of Group Psychotherapists Inc. -

Clinical Placement Office (CPO) [email protected] Level 3, 2-6 Bowes Street, Phillip, ACT 2606
Clinical Placement Office (CPO) [email protected] Level 3, 2-6 Bowes Street, Phillip, ACT 2606 Nursing & Midwifery Ward Contact List Canberra Health Services Switchboard – (02) 5124 2222 Students should contact either the Clinical Nurse Consultant (CNC) or Clinical Development Nurse (CDN) on the relevant telephone numbers below. Work area Contact number Notes Medical & Surgery Ward 10A – General surgery 512 42562 / 42236 Ward 9A - Gastroenterology 512 42337 / 42338 Ward 9B – Neurosurgery 512 42631 / 42632 Ward 8A – Haemodialysis 512 43362 / 43363 Ward 7B – General Medicine Unit (GMU) 512 42449 / 42275 Ward 7A – Stroke/Neurology 512 42535 / 42857 Ward 6A – Medical Cardiothoracic/Respiratory/ 512 42731 / 42732 Endocrinology/Rheumatology Ward 6B - Surgical Cardiothoracic/Urology/Vascular 512 42751 / 42653 Ward 5A - Orthopaedics 512 47905 / 47907 Ward 5B – General surgery/ENT 512 47918 / 47919 Ward 4B – Renal Medicine 512 42949 / 42938 Aged Care Ward 11A – Acute care elderly 512 42773 / 42770 Ward 11B – Sub acute aged care 512 42434 / 42435 Canberra Region Cancer Centre (CRCC) Ward 14A – Haematology Ward 512 42233 / 42351 Ward 8B* – Medical Oncology Ward 512 48611 / 48613 *Previously on 14B Canberra Region Cancer Centre (CRCC) 512 48444 Bldg 19 Switchboard Medical Oncology Day Unit 512 48457 Bldg 19, Level 4 Haematology Day Unit 512 44275 Bldg 19, Level 4 Immunology Day Unit 512 48457 Bldg 19, Level 4 Cancer Specialist Nurses 0412 502 010 Bldg 19, Level 5 CRCC Outpatient Department (OPD) 512 43510 Bldg 19, Level 2 Rapid Assessment Unit (RAU) -

National Capital Authority - Public Submission
NATIONAL CAPITAL AUTHORITY - PUBLIC SUBMISSION Joint Standing Committee on the National Capital and External Territories Inquiry into provision of amenity within the Parliamentary Triangle The role of the National Capital Authority 1. The National Capital Authority (NCA) manages the Australian Government’s continuing interest in Canberra as the National Capital. 2. The National Capital and Seat of Government is the legislative, judicial, administrative, executive, ceremonial and symbolic centre of the nation. Planning in the Central National Area 3. The Central National Area, which includes the Parliamentary Zone and its setting and the main diplomatic sites and national institutions, is the heart of the National Capital. 4. The NCA has direct responsibility for all aspects of planning and development approval in the Central National Area. 5. The planning system prescribes permitted land uses. Land Management and lease administration 6. Responsibility for land management and lease administration within the Central National Area is shared by the ACT Government, the Department of Finance and Deregulation (DoFD) and the NCA. 7. Each land manager, either directly or via leases they issue, is responsible for the extent to which they constrain, allow or mandate particular land uses from within those permitted under the planning system. 8. Land management decisions cannot be inconsistent with the planning system. 1 The changing nature of the working environment in the Parliamentary Triangle 9. The terms ‘Parliamentary Triangle’ and ‘National Triangle’ formally refer to the areas bounded by Commonwealth, Kings and Constitution Avenues. 10. The Triangle, however named, does not include the areas of Barton and Acton. 11. Given recent community debate about the provision of services and amenities in broader parts of the Central National Area, this submission has been prepared with reference to the Central National Areas of Parkes, Barton, Russell and Acton. -

100-Volunteer-Stories-Web-Version.Pdf
100 Volunteer STORIES www.volunteeringact.org.au © Volunteering ACT Inc 2013. December 2013 Celebrating the contribution of Canberra’s Volunteers Proudly sponsored by Beyond Bank Australia FOREWORD BY MAUREEN CANE Chief Executive Officer of Volunteering ACT Australia’s capital has a proud record for volunteering since it was founded. Services to the swiftly growing population were often first provided by volunteers. Volunteering is now in Canberra’s DNA, with many of her citizens, young and old and from all walks of life, actively volunteering at any one time. This book tells some of their stories — of care for others, of courage, of generosity and of application of skills and expertise. The stories are a testimony to the excellent work of Canberra’s community-based organisations and to the selfless contribution of individuals towards the general good. One such story, of the generous application of expertise, is from Sarah Wilson, the volunteer who compiled this book for Volunteering ACT! All the stories are a fitting legacy for Canberra’s Centenary year and engender a sure and certain confidence that our Maureen Cane, Volunteering ACT CEO future is in good hands. AUSTRALIA’S CAPITAL has a proud record for volunteering since it was founded. Services to the swiftly growing population were often firstprovided by volunteers. FOREWORD BY MARY PORTER Member of the ACT Legislative Assembly and Founder of Volunteering ACT As we all know the ACT has one of the highest rates of volunteering in Australia. When I arrived in the ACT in 1977 I was introduced to the communities training program that was sponsored by the Mental Health arm of the, then, Health Commission and supported by ACTOSS & Lifeline ACT. -

List of Accredited Chest Clinics (By State)
ACT Health Clinical Placement Office 2016 List of Accredited Chest clinics (By State) ACT Canberra Hospital TB Services Department of Contact: 02 6244 2066/ 02 6244 2702 Thoracic Medicine The Canberra Hospital PO Box 11 Woden ACT 2606 New South Wales Central Coast Local Health District Gosford Hospital Contact: TB Prevention & Control Service (Chest Clinic) Appt: 4320 3388 PO Box 361 Gosford NSW 2250 Only Mon 8.30-3; Tues & Fri 8.30-4 Illawarra and Shoalhaven LHD The Wollongong Hospital Contact: 4253 4138 Department of Respiratory Medicine Crown Street Wollongong NSW 2500 Nepean Blue Mountains Local Health District Nepean Hospital Contact: 4734 2536 Chest Clinic Outpatients Department PO Box 63 Penrith NSW 2751 Northern Sydney Local Health District Hornsby Ku-ring-gai Hospital Contact: 9477 9318 Palmerston Road Hornsby NSW 2077 New Royal North Shore Hospital Contact: 9926 7905 Chest Clinic Level 8, Dept. of Respiratory Medicine St Leonards NSW 2065 Manly District Hospital Contact: 9976 9542 Chest Clinic Manly NSW 2095 South Eastern Sydney LHD - Northern Network Prince of Wales Hospital Contact: 9382 4643/ 9382 4672 Department of Respiratory Medicine Level 2 Dickinson Building Barker Street Randwick NSW 2031 Sydney Hospital Contact: 9382 7535 Chest Clinic Macquarie Street Sydney NSW 2000 St. Vincent's Hospital Contact: 8382 3150 Heart-Lung Ambulatory Care Level 4, Xavier Building 390 Victoria Street Darlinghurst NSW 2010 South Eastern Sydney LHD - Central Network ACT Health Clinical Placement Office 2016 St George Hospital Contact: 9113 -

Canberra College Students Qualifying for This Honour
Principal’s Report - AUGUST 2015 I I welcome you all back to the business end of the year, particularly for our Year 12 students. I trust that all students returned rested and revitalised for the semester ahead. At our first week assembly I talked about the importance of feedback as it has two purposes; summative performance and also suggestions for improving performance. Last semester every student received a formal written summative report. This complements the feedback that students received throughout the term through the variety of learning and assessment activities as well as performance in the exams. Seeking and giving feedback also improves the performance of both teacher and student. Parent, teacher and student feedback along with performance data for the college collected over the past 4 years has now been analysed by an external panel as part of the review and validation process. The college validation report will be published before the end of term 3 and will contain a series of commendations and recommendations which will inform our planning for the next 4 years. I thank the students, staff and parents who contributed to the panels deliberations. In the next few weeks student, teachers and parents will be asked to complete the annual online school satisfaction survey. This is an important guide for us in our planning for whole college improvement. Please make the time complete this survey as we value your input and suggestions. Our staff and students continue to excel in a variety of areas. I acknowledge and congratulate; Jim Phillips who has won a promotion to Executive Teacher Science and PE at Lake Tuggeranong College. -

Integrating the Canberra Hospital ED with After Hours Primary Health Care Services – Final Report
Integrating the Canberra Hospital ED with after hours primary health care services – Final Report Capital Health Network 14 May 2018 Copyright & confidentiality: No part of this publication may be reproduced, stored in a retrieval system, translated, transcribed or transmitted in any form, or by any means manual, electric, electronic, electromagnetic, mechanical, chemical, optical, or otherwise, without the prior explicit written permission of Capital Health Network. © Nous Group Nous Group | Integrating the Canberra Hospital ED with after hours primary health care services – Final Report | 14 May 2018 | i | Contents 1 Executive Summary ...................................................................................................................................................................... 2 2 Introduction ..................................................................................................................................................................................... 5 3 Business Case .................................................................................................................................................................................. 8 4 Proposed interventions ........................................................................................................................................................... 13 5 Recommended interventions ............................................................................................................................................... -

June 2019 Newsletter
JUNE 2019 NEWSLETTER Principal’s Update Semester 1 at Canberra College has been a very positive time as the engagement of the college with the community continues to impress. Evidence of this includes the magnificent Woden Square Artspace Photography project through to the academic prestige of the ANU Maths Competition Runner-up result. Reflecting a tradition of highly capable student mathematicians, the college foyer trophy cabinet contains many trophies from the ANU Maths Competition, Including the overall winner in 2017. Final assessments for approximately 350 classes have been completed and student report writing has commenced. Please note that reports will be emailed to parents/carers and students on Friday 5 July. It was at the Board meeting this week that a decision was supported to also include all students in the email distribution of their reports. Previously, students would carry printed reports home in their schoolbags and deliver them to parents/carers. This practice also acknowledges that reports are written to students and contain feedback they should reference to continuously improve their performance. Student reports are valuable in reviewing student learning and pathways, and we encourage all parents and carers to discuss the reports at home. On the topic of feedback, all students are encouraged to complete a short online survey for each unit of study they undertake. This practice is highly valued by teachers as a way they too can use student feedback to reflect on their craft as educators and seek ways to strengthen their pedagogy and areas of practice. During this time College teachers are also required to prepare their Board of Senior Secondary Studies (BSSS) Unit Moderation Portfolios for submission this semester. -

1 October 1998
Annual report of the Australian Meningococcal Surveillance Programme, 1997 The Australian Meningococcal Surveillance Programme 1 Abstract The National Neisseria Network (NNN) has undertaken meningococcal isolate surveillance by means of a collaborative laboratory based initiative since 1994. The phenotype (serogroup, serotype and serosubtype) and antibiotic susceptibility of 343 isolates of Neisseria meningitidis from invasive cases of meningococcal disease were determined in 1997. Ninety six percent of the invasive isolates were serogroup B or C. Serogroup B strains predominated in all States and Territories and were isolated from sporadic cases of invasive disease. Phenotypes B:4:P1.4 and B:15:P1.7 were prominent. Serogroup C isolates were most often encountered in New South Wales, especially in adolescents and young adults, and in that State were nearly as numerous as serogroup B strains. C:2a:P1.5 was the most frequently encountered phenotype and C:2b:P1.2 strains were also distributed widely. A number of clusters of cases of serogroup C disease were noted, mainly with phenotype C:2a:P1.5. About three quarters of all isolates showed decreased susceptibility to the penicillin group of antibiotics (MIC 0.06 to 0.5 mg/L). Three isolates showed reduced susceptibility to rifampicin and one was chloramphenicol resistant. Commun Dis Itell 1998;22:205-211. Introduction of disease in an individual patient, and the patterns of the infection within a community may Invasive meningococcal diseases, manifested be materially altered by the characteristics of the mainly as bacteraemia and/or meningitis, remain infecting organism. The public health response to a conspicuous cause of morbidity and mortality in an outbreak or cluster of cases is also influenced 1 Australia and in 1997 attracted considerable by certain features of the invasive meningococci, ISSN 0725-3141 public attention. -

The Australian Guidelines for the Prevention and Control of Infection
AUSTRALIAN GUIDELINES FOR THE Prevention and Control of Infection in Healthcare WORKING TO BUILD A HEALTHY AUSTRALIA © Australian Government 2010 Electronic documents This work is copyright. You may download, display, print and reproduce this material in unaltered form only (retaining this notice) for your personal, non-commercial use, or use within your organisation. Apart from any use as permitted under the Copyright Act 1968, all other rights are reserved. Requests for further authorisation should be directed to the Commonwealth Copyright Administration, Attorney-General’s Department, Robert Garran Offices, National Circuit, Canberra, ACT, 2600 or posted at www.ag.gov.au/cca. ISBN Online: 1864965223 © Australian Government 2010 Paper-based publication This work is copyright. Apart from any use permitted under the Copyright Act 1968, no part may be reproduced by any process without written permission from the Commonwealth available from the Attorney-General’s Department. Requests and inquiries concerning reproduction and rights should be addressed to the Commonwealth Copyright Administration, Attorney-General’s Department, Robert Garran Offices, National Circuit, Canberra, ACT, 2600 or posted at www.ag.gov.au/cca. ISBN Print: 1864965282 This document should be cited as: NHMRC (2010) Australian Guidelines for the Prevention and Control of Infection in Healthcare. Commonwealth of Australia. Acknowledgements Infection Control Guidelines Steering Committee The production of a document such as this requires a considerable effort over a long