Ambroz 1997.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Volcanic Gases and Aerosols Guidelines Introduction
IVHHN Gas Guidelines www.ivhhn.org/gas/guidelines.html Volcanic Gases and Aerosols Guidelines The following pages contain information relating to the health hazards of gases and aerosols typically emitted during volcanic activity. Each section outlines the properties of the emission; its impacts on health; international guidelines for concentrations; and examples of concentrations and effects in volcanic contexts, including casualties. Before looking at the emissions data, we recommend that you read the general introduction to volcanic gases and aerosols first. A glossary to some of the terms used in the explanations and guidelines is also provided at the end of this document. Introduction An introduction to the aims and purpose of the Gas and Aerosol Guidelines is given here, as well as further information on international guideline levels and the units used in the website. A brief review of safety procedures currently implemented by volcanologists and volcano observatories is also provided. General Introduction Gas and aerosol hazards are associated with all volcanic activity, from diffuse soil gas emissions to 2- plinian eruptions. The volcanic emissions of most concern are SO2, HF, sulphate (SO4 ), CO2, HCl and H2S, although, there are other volcanic volatile species that may have human health implications, including mercury and other metals. Since 1900, there have been at least 62 serious volcanic-gas related incidents. Of these, the gas-outburst at Lake Nyos in 1986 was the most disastrous, causing 1746 deaths, >845 injuries and the evacuation of 4430 people. Other volcanic-gas related incidents have been responsible for more than 280 deaths and 1120 injuries, and contributed to the evacuation or ill health of >53,700 people (Witham, in review). -

Historic Fire Lookouts? the View and the Solitude Can’T Be Beat
ince shortly after the turn of the century, ¢ government personnel have stood guard Bonus Points 25 S over western lands. In Klamath, Lake, W ant to spend more time in one of the area’s and Modoc Country, fire lookouts open to the historic fire lookouts? The view and the solitude can’t be beat. public offer breathtaking views from the top of • Bald Butte Lookout Rental - Many historic area lookouts HISTORIC the world as well as a chance to visit history . are being made available as rustic vacation rentals. Reservations are required. For Bald Butte Lookout Rental, arly fire lookouts were simply scaffolds, and outdoor recreation activities including skiing and FIRE E snowmobiling, contact the Paisley Ranger District, attached precariously to trees and offering 541-943-3114. Bald Butte Lookout is available little shelter to early fire observers. year-round. • Hager Mountain Lookout Rental - While a little more LOOKOUTS ost surviving fire lookout towers, built in difficult to get to, this lookout offers the adventurous a M the 20’s and 30’s, are 14' by 14' structures breathtaking view year-round from over 7,000 feet. On a assembled from pre-manufactured kits and clear day you might see as far north as Mt. Hood, and south to Mt. Shasta. Contact the Silver Lake Ranger packed up to mountain peaks by truck or even District, 541-576-2107, for information and reservations. mule train. Windows offered a 360-degree view Reservations also can be made for the Fremont Point Cabin of the area for the occupant. All the comforts of perched on the edge of the massive escarpment west of home were available: wood stove, bed and Summer Lake. -

USGS USGS Geologic Investigations Series I-2569, Pamphlet
U.S. DEPARTMENT OF THE INTERIOR TO ACCOMPANY MAP I–2569 U.S. GEOLOGICAL SURVEY GEOLOGIC MAP OF UPPER EOCENE TO HOLOCENE VOLCANIC AND RELATED ROCKS OF THE CASCADE RANGE, OREGON By David R. Sherrod and James G. Smith INTRODUCTION volcanic-hosted mineral deposits and even to potential volcanic hazards. Since 1979, Earth scientists of the Geothermal Historically, the regional geology of the Cascade Research Program of the U.S. Geological Survey have Range in Oregon has been interpreted through recon carried out multidisciplinary research in the Cascade naissance studies of large areas (for example, Diller, Range. The goal of this research is to understand 1898; Williams, 1916; Callaghan and Buddington, the geology, tectonics, and hydrology of the Cas cades in order to characterize and quantify geothermal 1938; Williams, 1942, 1957; Peck and others, 1964). Early studies were hampered by limited access, resource potential. A major goal of the program generally poor exposures, and thick forest cover, which is compilation of a comprehensive geologic map of flourishes in the 100 to 250 cm of annual precipi the entire Cascade Range that incorporates modern tation west of the range crest. In addition, age control field studies and that has a unified and internally con was scant and limited chiefly to fossil flora. Since sistent explanation. This map is one of three in a series that shows then, access has greatly improved via a well-devel- oped network of logging roads, and isotopic ages— Cascade Range geology by fitting published and mostly potassium-argon (K-Ar)—have gradually solved unpublished mapping into a province-wide scheme some major problems concerning timing of volcan of rock units distinguished by composition and age; ism and age of mapped units. -

Upper Deschutes River · ·Basin Prehistory
Upper Deschutes River · ·Basin Prehistory: A Preliminary Examination of Flaked Stone Tools and Debitage Michael W. Taggart 2002 ·~. ... .. " .. • '·:: ••h> ·';'"' •..,. •.• '11\•.. ...... :f~::.. ·:·. .. ii AN ABSTRACT OF THE THESIS OF Michael W. Taggart for the degree of Master of Arts in Interdisciplinary Studies in Anthropology. Anthropology. and Geography presented on April 19. 2002. Title: Upper Deschutes River Basin Prehistory: A Preliminary Examination of Flaked Stone Tools and Debitage. The prehistory of Central Oregon is explored through the examination of six archaeological sites and two isolated finds from the Upper Deschutes River Basin. Inquiry focuses on the land use, mobility, technological organization, and raw material procurement of the aboriginal inhabitants of the area. Archaeological data presented here are augmented with ethnographic accounts to inform interpretations. Eight stone tool assemblages and three debitage assemblages are analyzed in order to characterize technological organization. Diagnostic projectile points recovered from the study sites indicate the area was seasonally utilized prior to the eruption of ancient Mt. Mazama (>6,845 BP), and continuing until the Historic period (c. 1850). While there is evidence of human occupation at the study sites dating to between >7,000- 150 B.P., the range of activities and intensity of occupation varied. Source characterization analysis indicates that eight different Central Oregon obsidian sources are represented at the sites. Results of the lithic analysis are presented in light of past environmental and social phenomena including volcanic eruptions, climate change, and human population movements. Chapter One introduces the key questions that directed the inquiry and defines the theoretical perspective used. Chapter Two describes the modem and ancient environmental context of study area. -

GY 111: Physical Geology
UNIVERSITY OF SOUTH ALABAMA GY 111: Physical Geology Lecture 9: Extrusive Igneous Rocks Instructor: Dr. Douglas W. Haywick Last Time 1) The chemical composition of the crust 2) Crystallization of molten rock 3) Bowen's Reaction Series Web notes 8 Chemical Composition of the Crust Element Wt% % of atoms Oxygen 46.6 60.5 Silicon 27.7 20.5 Aluminum 8.1 6.2 Iron 5.0 1.9 Calcium 3.6 1.9 Sodium 2.8 2.5 Potassium 2.6 1.8 Magnesium 2.1 1.4 All other elements 1.5 3.3 Crystallization of Magma http://myweb.cwpost.liu.edu/vdivener/notes/igneous.htm Bowen’s Reaction Series Source http://www.ltcconline.net/julian Igneous Rock Composition Source: http://hyperphysics.phy-astr.gsu.edu Composition Formation Dominant Silica content Temperature Minerals Ultramafic Very high Olivine, pyroxene Very low (<45%) Mafic High Olivine, pyroxene, low Ca-plagioclase Intermediate Medium Na-Plagioclase, moderate amphibole, biotite Felsic Medium-low Orthoclase, quartz, high (>65%) muscovite, biotite Igneous Rock Texture Extrusive Rocks (Rapid Cooling; non visible* crystals) Intrusive Rocks (slow cooling; 100 % visible crystals) *with a hand lens Igneous Rock Texture Igneous Rock Texture Today’s Agenda 1) Pyro-what? (air fall volcanic rocks) 2) Felsic and Intermediate Extrusive Rocks 3) Mafic Extrusive Rocks Web notes 9 Pyroclastic Igneous Rocks Pyroclastic Igneous Rocks Pyroclastic: Pyro means “fire”. Clastic means particles; both are of Greek origin. Pyroclastic Igneous Rocks Pyroclastic: Pyro means “fire”. Clastic means particles; both are of Greek origin. Pyroclastic rocks are usually erupted from composite volcanoes (e.g., they are produced via explosive eruptions from viscous, “cool” lavas) Pyroclastic Igneous Rocks Pyroclastic: Pyro means “fire”. -

Lower Sycan Watershed Analysis
Lower Sycan Watershed Analysis Fremont-Winema National Forest 2005 Lower Sycan River T33S,R12E,S23 Lower Sycan Watershed Analysis Table of Contents INTRODUCTION...................................................................................................................................... 1 General Watershed Area.....................................................................................................................................2 Geology and Soils.................................................................................................................................................5 Climate..................................................................................................................................................................6 STEP 1. CHARACTERIZATION OF THE WATERSHED ................................................................... 7 I. Watershed and Aquatics.................................................................................................................................7 Soils And Geomorphology...............................................................................................................................................10 Aquatic Habitat ................................................................................................................................................................10 II. Vegetation.....................................................................................................................................................12 -

Deep Carbon Emissions from Volcanoes Michael R
Reviews in Mineralogy & Geochemistry Vol. 75 pp. 323-354, 2013 11 Copyright © Mineralogical Society of America Deep Carbon Emissions from Volcanoes Michael R. Burton Istituto Nazionale di Geofisica e Vulcanologia Via della Faggiola, 32 56123 Pisa, Italy [email protected] Georgina M. Sawyer Laboratoire Magmas et Volcans, Université Blaise Pascal 5 rue Kessler, 63038 Clermont Ferrand, France and Istituto Nazionale di Geofisica e Vulcanologia Via della Faggiola, 32 56123 Pisa, Italy Domenico Granieri Istituto Nazionale di Geofisica e Vulcanologia Via della Faggiola, 32 56123 Pisa, Italy INTRODUCTION: VOLCANIC CO2 EMISSIONS IN THE GEOLOGICAL CARBON CYCLE Over long periods of time (~Ma), we may consider the oceans, atmosphere and biosphere as a single exospheric reservoir for CO2. The geological carbon cycle describes the inputs to this exosphere from mantle degassing, metamorphism of subducted carbonates and outputs from weathering of aluminosilicate rocks (Walker et al. 1981). A feedback mechanism relates the weathering rate with the amount of CO2 in the atmosphere via the greenhouse effect (e.g., Wang et al. 1976). An increase in atmospheric CO2 concentrations induces higher temperatures, leading to higher rates of weathering, which draw down atmospheric CO2 concentrations (Ber- ner 1991). Atmospheric CO2 concentrations are therefore stabilized over long timescales by this feedback mechanism (Zeebe and Caldeira 2008). This process may have played a role (Feulner et al. 2012) in stabilizing temperatures on Earth while solar radiation steadily increased due to stellar evolution (Bahcall et al. 2001). In this context the role of CO2 degassing from the Earth is clearly fundamental to the stability of the climate, and therefore to life on Earth. -
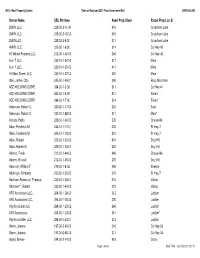
SBL Printkey Asmt Prop Class Parcel Prop Loc St Owner
NYS - Real Property System Town of Maryland 2021 Final Assesment Roll CRW/V4/L001 Owner Name SBL Printkey Asmt Prop Class Parcel Prop Loc St 2MNY, LLC, 228.00-2-11.00 910 Crumhorn Lake 2MNY, LLC, 228.00-2-15.00 910 Crumhorn Lake 2MNY,LLC, 228.00-2-8.00 311 Crumhorn Lake 4MNY, LLC, 229.00-1-6.00 314 Co Hwy 42 50 Willow Porperty LLC, 212.00-1-30.00 240 Co Hwy 42 6 on 7 LLC, 230.19-1-34.00 311 Main 6 on 7 LLC, 230.19-1-35.00 411 Main 91 Main Street, LLC, 230.19-1-37.00 481 Main Abel, James Otto 245.00-1-49.01 240 Ross Mountain ACD HOLDING CORP., 264.00-1-3.03 311 Co Hwy 41 ACD HOLDING CORP., 264.00-1-6.00 314 Bliven* ACD HOLDING CORP., 264.00-1-7.00 314 Bliven* Ackerson, Robert C. 230.20-1-17.00 210 East Ackerson, Robert C. 230.20-1-68.00 311 Main* Acosta, Pablo 229.00-1-60.00 270 Chaseville Adair, Frederick M. 245.10-1-17.01 270 St Hwy 7 Adair, Frederick M. 245.10-1-18.00 210 St Hwy 7 Adair, Robert 229.00-1-35.00 910 Dog Hill Adair, Robert G. 229.00-1-53.01 240 Dog Hill Adamo, Frank 212.00-1-44.00 240 Chaseville Adams, Russell 212.00-1-35.00 270 Dog Hill Adamski, William T. 279.00-1-8.03 240 Shelton Addorigio, Kimberly 230.00-1-20.00 210 St Hwy 7 Adolfsen-Robinson, Therese A. -

Insights Into the Recurrent Energetic Eruptions That Drive Awu Among the Deadliest Volcanoes on Earth
Insights into the recurrent energetic eruptions that drive Awu among the deadliest volcanoes on earth Philipson Bani1, Kristianto2, Syegi Kunrat2, Devy Kamil Syahbana2 5 1- Laboratoire Magmas et Volcans, Université Blaise Pascal - CNRS -IRD, OPGC, Aubière, France. 2- Center for Volcanology and Geological Hazard Mitigation (CVGHM), Jl. Diponegoro No. 57, Bandung, Indonesia Correspondence to: Philipson Bani ([email protected]) 10 Abstract The little known Awu volcano (Sangihe island, Indonesia) is among the deadliest with a cumulative death toll of 11048. In less than 4 centuries, 18 eruptions were recorded, including two VEI-4 and three VEI-3 eruptions with worldwide impacts. The regional geodynamic setting is controlled by a divergent-double-subduction and an arc-arc collision. In that context, the slab stalls in the mantle, undergoes an increase of temperature and becomes prone to 15 melting, a process that sustained the magmatic supply. Awu also has the particularity to host alternatively and simultaneously a lava dome and a crater lake throughout its activity. The lava dome passively erupted through the crater lake and induced strong water evaporation from the crater. A conduit plug associated with this dome emplacement subsequently channeled the gas emission to the crater wall. However, with the lava dome cooling, the high annual rainfall eventually reconstituted the crater lake and created a hazardous situation on Awu. Indeed with a new magma 20 injection, rapid pressure buildup may pulverize the conduit plug and the lava dome, allowing lake water injection and subsequent explosive water-magma interaction. The past vigorous eruptions are likely induced by these phenomena, a possible scenario for the future events. -

Volcano Hazards at Newberry Volcano, Oregon
Volcano Hazards at Newberry Volcano, Oregon By David R. Sherrod1, Larry G. Mastin2, William E. Scott2, and Steven P. Schilling2 1 U.S. Geological Survey, Hawaii National Park, HI 96718 2 U.S. Geological Survey, Vancouver, WA 98661 OPEN-FILE REPORT 97-513 This report is preliminary and has not been reviewed for conformity with U.S. Geological Survey editorial standards or with the North American Stratigraphic Code. Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the U.S. Government. 1997 U.S. Department of the Interior U.S. Geological Survey CONTENTS Introduction 1 Hazardous volcanic phenomena 2 Newberry's volcanic history is a guide to future eruptions 2 Flank eruptions would most likely be basaltic 3 The caldera would be the site of most rhyolitic eruptions—and other types of dangerously explosive eruptions 3 The presence of lakes may add to the danger of eruptions in the caldera 5 The most damaging lahars and floods at Newberry volcano would be limited to the Paulina Creek area 5 Small to moderate-size earthquakes are commonly associated with volcanic activity 6 Volcano hazard zonation 7 Hazard zone for pyroclastic eruptions 7 Regional tephra hazards 8 Hazard zone for lahars or floods on Paulina Creek 8 Hazard zone for volcanic gases 10 Hazard zones for lava flows 10 Large-magnitude explosive eruptions of low probability 11 Monitoring and warnings 12 Suggestions for further reading 12 Endnotes 13 ILLUSTRATIONS Plate 1. Volcano hazards at Newberry volcano, Oregon in pocket Figure 1. Index map showing Newberry volcano and vicinity 1 Figure 2. -

Explore!Outdoor, Indoor & Around Town Adventures In
Explore!Outdoor, Indoor & Around Town Adventures in A NATIONAL HERITAGE CORRIDOR www.thelastgreenvalley.org • TOLL FREE 866-363-7226 The Last Green Valley National Heritage Corridor - together we can care for it, enjoy it, EXPLORE! Table of Contents The Last Green Valley Map . 2 and pass it on. Accommodations . 4 Astronomy/Night Sky Views . 5 Bicycling & Mountain Biking . 6 Welcome Boating and/or Fishing . 8 Are you a modern Camping . 14 Chambers/Economic Development . 16 day Explorer? You can Disc Golf . 19 be! Discover the natural Education . 20 beauty of The Last Green Farms/Orchards/Nurseries . 21 Valley National Heritage Hiking, Walking & Strolling Trails . 24 Corridor (35 towns in Horseback Riding & Horse Camping . 36 northeast CT and south Hunting . 38 Labyrinths/Mazes . 39 central MA). Find wonder Letterboxing & Geocaching . 40 in the waterfalls, the fishing MORE! Outdoor Activities & Sites holes, the hilltops, and the Proud Supporters/Creators of Outdoor Fun . 41 farms. Hear stories from the Even More Outdoor Activities & Sites . 42 past, sip wine in a vineyard, Museums & Historic Sites . 44 Nonprofits . 48 shop til you drop, and savor Paddling . 50 local foods. Kayak, backpack, Retail - Arts, Antiques & Uniques . 56 pick an apple, or carve a Scenic Overlooks & Views . 58 pumpkin. Savor farm fresh Service Businesses food, photograph bald Medical Emergency Facilities . 60 eagles in flight, or gaze at General Services . 61 Skate Parks . 65 the stars. Explore! will help State & Federal Parks & Forests Chart . 66 you delve into every inch of State & Federal Parks & Forests Map . 70 The Last Green Valley. We State & Federal Parks & Forests Descriptions . 72 will increase your capacity Swimming & Scuba Diving . -

A Portion of South-Central Oregon
DEPARTMENT OF THE INTERIOR UNITED STATES GEOLOGICAL SURVEY GEORGE OTIS SMITH, DIRECTOR WATER-SUPPLY PAPER 220 GEOLOGY AND WATER RESOURCES OF A PORTION OF SOUTH-CENTRAL OREGON BY GERALD A. WARING WASHINGTON GOVERNMENT FEINTING OFFICE 1908 DEPARTMENT OF THE INTERIOR UNITED STATES GEOLOGICAL SURVEY GEORGE OTIS SMITH, DIKEOTOK WATER-SUPPLY PAPER 22O GEOLOGY AND WATER RESOURCES OF A PORTION OF SOUTH-CENTRAL OREGON BY GERALD A. WARING WASHINGTON GOVERNMENT PRINTING OFFICE 1908 CONTENTS. Vage. Introduction.............................................................. 7 Objects of reconnaissance.............................................. 7 Area examined........................................................ 7 Acknowledgements..................................................... 8 Previous study......................................................... 8 Geography................................................................. 9 General features....................................................... 9 Topography............................................................. 9 Mountains........................................................ 9 Scarps.............................................................. 9 Minor features..................................................... 10 Lakes.................................................................. 11 Character of the lakes................................................ 12 Alkalinity........................................................ 12 . Climate...............................................................