(Centrarchidae) and Pygmy Sunfishes (Elassomatidae) in the Netherlands
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

North Carolina Wildlife Resources Commission Gordon Myers, Executive Director
North Carolina Wildlife Resources Commission Gordon Myers, Executive Director March 1, 2016 Honorable Jimmy Dixon Honorable Chuck McGrady N.C. House of Representatives N.C. House of Representatives 300 N. Salisbury Street, Room 416B 300 N. Salisbury Street, Room 304 Raleigh, NC 27603-5925 Raleigh, NC 27603-5925 Senator Trudy Wade N.C. Senate 300 N. Salisbury Street, Room 521 Raleigh, NC 27603-5925 Dear Honorables: I am submitting this report to the Environmental Review Committee in fulfillment of the requirements of Section 4.33 of Session Law 2015-286 (H765). As directed, this report includes a review of methods and criteria used by the NC Wildlife Resources Commission on the State protected animal list as defined in G.S. 113-331 and compares them to federal and state agencies in the region. This report also reviews North Carolina policies specific to introduced species along with determining recommendations for improvements to these policies among state and federally listed species as well as nonlisted animals. If you have questions or need additional information, please contact me by phone at (919) 707-0151 or via email at [email protected]. Sincerely, Gordon Myers Executive Director North Carolina Wildlife Resources Commission Report on Study Conducted Pursuant to S.L. 2015-286 To the Environmental Review Commission March 1, 2016 Section 4.33 of Session Law 2015-286 (H765) directed the N.C. Wildlife Resources Commission (WRC) to “review the methods and criteria by which it adds, removes, or changes the status of animals on the state protected animal list as defined in G.S. -

Indiana Species April 2007
Fishes of Indiana April 2007 The Wildlife Diversity Section (WDS) is responsible for the conservation and management of over 750 species of nongame and endangered wildlife. The list of Indiana's species was compiled by WDS biologists based on accepted taxonomic standards. The list will be periodically reviewed and updated. References used for scientific names are included at the bottom of this list. ORDER FAMILY GENUS SPECIES COMMON NAME STATUS* CLASS CEPHALASPIDOMORPHI Petromyzontiformes Petromyzontidae Ichthyomyzon bdellium Ohio lamprey lampreys Ichthyomyzon castaneus chestnut lamprey Ichthyomyzon fossor northern brook lamprey SE Ichthyomyzon unicuspis silver lamprey Lampetra aepyptera least brook lamprey Lampetra appendix American brook lamprey Petromyzon marinus sea lamprey X CLASS ACTINOPTERYGII Acipenseriformes Acipenseridae Acipenser fulvescens lake sturgeon SE sturgeons Scaphirhynchus platorynchus shovelnose sturgeon Polyodontidae Polyodon spathula paddlefish paddlefishes Lepisosteiformes Lepisosteidae Lepisosteus oculatus spotted gar gars Lepisosteus osseus longnose gar Lepisosteus platostomus shortnose gar Amiiformes Amiidae Amia calva bowfin bowfins Hiodonotiformes Hiodontidae Hiodon alosoides goldeye mooneyes Hiodon tergisus mooneye Anguilliformes Anguillidae Anguilla rostrata American eel freshwater eels Clupeiformes Clupeidae Alosa chrysochloris skipjack herring herrings Alosa pseudoharengus alewife X Dorosoma cepedianum gizzard shad Dorosoma petenense threadfin shad Cypriniformes Cyprinidae Campostoma anomalum central stoneroller -
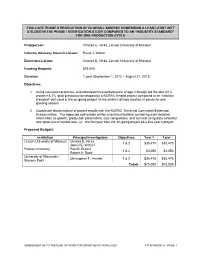
Bluegill Diet Verification Project Outline Final W Letter of Support.Pdf
EVALUATE PHASE II PRODUCTION OF BLUEGILL SUNFISH COMPARING A LEAST-COST DIET UTILIZED IN THE PHASE I VERIFICATION STUDY COMPARED TO AN “INDUSTRY STANDARD” FOR ONE PRODUCTION CYCLE Chairperson: Charles E. Hicks, Lincoln University of Missouri Industry Advisory Council Liaison: Paula J. Moore Extension Liaison: Charles E. Hicks, Lincoln University of Missouri Funding Request: $75,000 Duration: 1 year (September 1, 2012 – August 31, 2013) Objectives: 1. Using consistent protocols, evaluate/determine performance of age-2 bluegill fed the diet (41% protein/<8.3% lipid) previously developed by a NCRAC funded project compared to an “industry standard” diet used in the on-going project at two distinct latitude location in ponds for one growing season. 2. Coordinate dissemination of project results with the NCRAC Technical Committee/Extension Subcommittee. The expected deliverable will be a technical bulletin containing such detailed information as growth, production parameters, size composition, and survival using data collected over grow out to market size; i.e., the first year from the on-going project plus this year’s project. Proposed Budgets: Institution Principal Investigators Objectives Year 1 Total Lincoln University of Missouri Charles E. Hicks 1 & 2 $35,475 $35,475 James E. Wetzel Purdue University Paul B. Brown 1 & 2 $3,050 $3,050 Robert A. Rode University of Wisconsin - Christopher F. Hartleb 1 & 2 $36,475 $36,475 Stevens Point Totals $75,000 $75,000 \AMENDMENT #2 TO THE PLAN OF WORK FOR GRANT #2010-38500-20929 ATTACHMENT A - PAGE 1 TABLE OF CONTENTS SUMMARY OVERVIEW (PARTICIPANTS, OBJECTIVES, AND PROPOSED BUDGETS) ....................... 1 JUSTIFICATION ........................................................................................................................................... 3 RELATED CURRENT AND PREVIOUS WORK ......................................................................................... -

Albemarle-Pamlico Estuarine Study
Report No. 90-16 FOOD AND FEEDING OF YOUNG FINFISH SPECIES IN THE LOWER ROANOKE RIVER, BATCHELOR BAY, AND WESTERN ALBEMARLE SOUND, NORTH CAROLINA, 1982-1988 Volume I - Text Roger A. Rulifson. John E. Cooper, Donald W. Stanley, Marsha E. Shepherd, Scott F. Wood, and Deborah A. Daniel l .. .;' ~ ·~ - -~ ' ' . ' u r: ,' . , . C1 h· !1. :.· •. II j 509;F68 )\y}., 1 • I It ! I 1 f 'f I I ., I ' I ' 1994 I ALBEMARLE-PAMLICO ESTUARINE STUDY NC O..patlment ot Environmental Environment, Heollh, Protection Agency and Nolurol Resources =~81~ Notional Esluory Program Food and Feeding of Young Finfish Species in the Lower Roanoke River, Batchelor Bay, and Western Albemarle Sound, North Carolina, 1982-1988 Volume I - Text By 1 2 1 1 2 Roger A. Rulifson • , John E. Cooper , Donald W. Stanley • , Marsha E. Shepherd3, Scott F. Wood 1•2, and Deborah A. Daniel2 1/nstitute for Coastal and Marine Resources 2Department ofBiology 3Academic Computing East Carolina University Greenville, NC 27858-4353 (ICMR Contribution Series, No. ICMR-93-04) The research on which the report is based was financed, in part, by the United States Environmental Protection Agency and the North Carolina Department of Environment, Health, and Natural Resources, through the Albemarle-Pamlico Study. Additional support was provided by the U.S. Department of the Interior, Fish and Wildlife Service, under the Wallop-Breaux Amendment to the Sport Fish Restoration Act. Contents of the publication do not necessarily reflect the views and policies of the United States Environmental Protection Agency, the North Carolina Department of Environment, Health, and Natural Resources, nor does mention of trade names or commercial products constitute their endorsement by the United States or North Carolina governments. -

2017 Cerimele Nicole Thesis.Pdf (4.200Mb)
UNIVERSITY OF OKLAHOMA GRADUATE COLLEGE EARLY MIDDLE ARCHAIC PLACEMAKING: A FAUNAL ANALYSIS OF THREE PIT DEPOSITS FROM SILVER GLEN SPRINGS (8LA1), FLORIDA A THESIS SUBMITTED TO THE GRADUATE FACULTY in partial fulfillment of the requirements for the Degree of MASTER OF ARTS By NICOLE GRACE CERIMELE Norman, Oklahoma 2017 EARLY MIDDLE ARCHAIC PLACEMAKING: A FAUNAL ANALYSIS OF THREE PIT DEPOSITS FROM SILVER GLEN SPRINGS (8LA1), FLORIDA A THESIS APPROVED FOR THE DEPARTMENT OF ANTHROPOLOGY BY _______________________________ Dr. Asa Randall, Chair ______________________________ Dr. Leland Bement ______________________________ Dr. Patrick Livingood © Copyright by NICOLE GRACE CERIMELE 2017 All Rights Reserved. DEDICATION To my husband, Josh, for welcoming me to the United States. Without his love and support this thesis would not have been possible. Also, to our son, expected December 2017. ACKNOWLEDGEMENTS First, I would like to give many thanks to my thesis committee, Dr. Asa Randall, Dr. Leland Bement, and Dr. Patrick Livingood, for their extensive guidance during the past two and a half years. I would also like to thank Department of Anthropology for allowing the opportunity to receive both the Morris E. Opler Memorial Scholarship and the Pat Gilman and Paul Minnis Scholarship, which allowed me to go to Florida to access the zooarchaeological collections at the Florida Museum of Natural History. I would especially like to thank Dr. Katherine Emery for providing access to the FMNH collections and Meggan Blessing, Nicole Cannarozzi, and Dr. Joshua Goodwin for their patience and time in educating me on the finer details of St. Johns riverine zooarchaeology. I would also like to thank both Dr. -

Tennessee Fish Species
The Angler’s Guide To TennesseeIncluding Aquatic Nuisance SpeciesFish Published by the Tennessee Wildlife Resources Agency Cover photograph Paul Shaw Graphics Designer Raleigh Holtam Thanks to the TWRA Fisheries Staff for their review and contributions to this publication. Special thanks to those that provided pictures for use in this publication. Partial funding of this publication was provided by a grant from the United States Fish & Wildlife Service through the Aquatic Nuisance Species Task Force. Tennessee Wildlife Resources Agency Authorization No. 328898, 58,500 copies, January, 2012. This public document was promulgated at a cost of $.42 per copy. Equal opportunity to participate in and benefit from programs of the Tennessee Wildlife Resources Agency is available to all persons without regard to their race, color, national origin, sex, age, dis- ability, or military service. TWRA is also an equal opportunity/equal access employer. Questions should be directed to TWRA, Human Resources Office, P.O. Box 40747, Nashville, TN 37204, (615) 781-6594 (TDD 781-6691), or to the U.S. Fish and Wildlife Service, Office for Human Resources, 4401 N. Fairfax Dr., Arlington, VA 22203. Contents Introduction ...............................................................................1 About Fish ..................................................................................2 Black Bass ...................................................................................3 Crappie ........................................................................................7 -

Endangered Species
FEATURE: ENDANGERED SPECIES Conservation Status of Imperiled North American Freshwater and Diadromous Fishes ABSTRACT: This is the third compilation of imperiled (i.e., endangered, threatened, vulnerable) plus extinct freshwater and diadromous fishes of North America prepared by the American Fisheries Society’s Endangered Species Committee. Since the last revision in 1989, imperilment of inland fishes has increased substantially. This list includes 700 extant taxa representing 133 genera and 36 families, a 92% increase over the 364 listed in 1989. The increase reflects the addition of distinct populations, previously non-imperiled fishes, and recently described or discovered taxa. Approximately 39% of described fish species of the continent are imperiled. There are 230 vulnerable, 190 threatened, and 280 endangered extant taxa, and 61 taxa presumed extinct or extirpated from nature. Of those that were imperiled in 1989, most (89%) are the same or worse in conservation status; only 6% have improved in status, and 5% were delisted for various reasons. Habitat degradation and nonindigenous species are the main threats to at-risk fishes, many of which are restricted to small ranges. Documenting the diversity and status of rare fishes is a critical step in identifying and implementing appropriate actions necessary for their protection and management. Howard L. Jelks, Frank McCormick, Stephen J. Walsh, Joseph S. Nelson, Noel M. Burkhead, Steven P. Platania, Salvador Contreras-Balderas, Brady A. Porter, Edmundo Díaz-Pardo, Claude B. Renaud, Dean A. Hendrickson, Juan Jacobo Schmitter-Soto, John Lyons, Eric B. Taylor, and Nicholas E. Mandrak, Melvin L. Warren, Jr. Jelks, Walsh, and Burkhead are research McCormick is a biologist with the biologists with the U.S. -

Chapter 10 Plan Review and Revision
Chapter 10 Plan Review and Revision PLAN REVIEW AND REVISION Table of Contents Introduction ................................................................................................................................ 10-1 Maryland SWAP Review Schedule ........................................................................................... 10-2 Maryland SWAP and the State Wildlife Grant Program ........................................................... 10-3 State Wildlife Grant Projects ..................................................................................................... 10-3 Conservation Planning ........................................................................................................ 10-6 Technical Assistance .......................................................................................................... 10-8 Inventory, Monitoring, and Research ............................................................................... 10-10 Mammals ..................................................................................................................... 10-10 Birds............................................................................................................................. 10-11 Reptiles and Amphibians ............................................................................................. 10-13 Invertebrates ................................................................................................................ 10-16 Multiple Animal Taxa ................................................................................................. -

Summary Report of Freshwater Nonindigenous Aquatic Species in U.S
Summary Report of Freshwater Nonindigenous Aquatic Species in U.S. Fish and Wildlife Service Region 4—An Update April 2013 Prepared by: Pam L. Fuller, Amy J. Benson, and Matthew J. Cannister U.S. Geological Survey Southeast Ecological Science Center Gainesville, Florida Prepared for: U.S. Fish and Wildlife Service Southeast Region Atlanta, Georgia Cover Photos: Silver Carp, Hypophthalmichthys molitrix – Auburn University Giant Applesnail, Pomacea maculata – David Knott Straightedge Crayfish, Procambarus hayi – U.S. Forest Service i Table of Contents Table of Contents ...................................................................................................................................... ii List of Figures ............................................................................................................................................ v List of Tables ............................................................................................................................................ vi INTRODUCTION ............................................................................................................................................. 1 Overview of Region 4 Introductions Since 2000 ....................................................................................... 1 Format of Species Accounts ...................................................................................................................... 2 Explanation of Maps ................................................................................................................................ -

Fine Structure of the Retina of Black Bass, Micropterus Salmoides (Centrarchidae, Teleostei)
Histol Histopathol (1999) 14: 1053-1065 Histology and 001: 10.14670/HH-14.1053 Histopathology http://www.hh.um.es From Cell Biology to Tissue Engineering Fine structure of the retina of black bass, Micropterus salmoides (Centrarchidae, Teleostei) M. Garcia and J. de Juan Department of Biotechnology, University of Alicante, Alicante, Spain Summary. The structure of light- and dark-adapted 1968), 4) regular cone mosaics (Wagner, 1978), 5) the retina of the black bass, Micropterus salmoides has been existence of a well developed retinal tapetum in studied by light and electron microscopy. This retina nocturnal and bottom-living species (Wagner and Ali, lacks blood vessels at all levels. The optic fiber layer is 1978), 6) presence of foveae or areas with an increase in divided into fascicles by the processes of Muller cells photoreceptors and other neurons (Wagner, 1990), and 7) and the ganglion cell layer is represented by a single row a marked synaptic plasticity as is demonstrated by the of voluminous cells. The inner nuclear layer consists of formation of spinules (Wagner, 1980) during light two layers of horizontal cells and bipolar, amacrine and adaptation and their disappearance during dark interplexiform cells. In the outer plexiform layer we adaptation, and others. In summary, these features observed the synaptic terminals of photoreceptor celis, demonstrate the importance of vision in the mode of life rod spherules and cone pedicles and terminal processes and survival of the species. of bipolar and horizontal cells. The spherules have a Micropterus salmoides, known as black bass, is a single synaptic ribbon and the pedicles possess multiple member of the family Centrarchidae (Order Perciformes) synaptic ribbons. -

Winter Biology of Centrarchid Fishes C
Chapter 9 Winter biology of centrarchid fishes C. D. Suski and M. S. Ridgway 9.1 Introduction Temperate latitudes experience a predictable annual cycle of alternating warm and cold periods that can result in below freezing conditions, ice cover, and alterations to aquatic habitats that persist for a substantial portion of a year. Winter represents a very interesting and challenging time of the year that exerts a strong selective pressure on individual survival, community structure, and year class strength for centrarchid fishes. Despite the impact of this time on both individuals and populations, we are only beginning to comprehend how this period of the year can influence centrarchid fishes. The purpose of this chapter is to summarize the current literature that defines the ecological, behavioral, and physio- logical alterations experienced by centrarchid fishes both prior to and during winter. Because of the paucity of information on winter biology of centrarchid fishes, this chapter has been written in a general format whereby studies of different centrarchid fishes have been pooled to identify trends that exist across the entire family. Where appropriate, exceptions to these general trends have been noted. A general over-arching question does emerge from work to date despite the lack of broad research coverage in many areas of centrarchid winter biology: What physiological and ecological changes occur to ensure survival prior to and during a period of reduced energy intake? 9.2 Definition of “winter” We define “winter” as the period of the year between the autumnal equinox and prior to the onset of spawning in centrarchid fishes. -

New Jersey Anglers Know a Lot About Fishing the Garden State. Most Avid Fishermen Can Distinguish a Largemouth from a Smallmouth Bass
TrueNew Jersey NativesBy SHAWN CROUSE Principal Fisheries Biologist Photos by author New Jersey anglers know a lot about fishing the Garden State. Most avid fishermen can distinguish a largemouth from a smallmouth bass. Many of us catch our limit of stocked trout on opening day while others have wet enough lines to have mastered techniques for catching lake trout, walleye or muskie. But many anglers may not know that each of these species, including most of our popular gamefish, were introduced to our state for recreational purposes. That’s right, northern pike, channel catfish, rainbow and brown trout, hybrid stripers, common carp, crappie and even bluegill are not native to New Jersey. bluespotted sunfish Of the nearly 100 freshwater fish species that swim cal region in which it evolved, whereas non-native in our waters, only 65 of them are native. The term species have been dispersed by humans (inten- native is often misused to describe an individual tionally or unintentionally) beyond their original fish that was born in the wild. The most common geographical region. misuse among anglers is when we claim to catch With the exception of our native sportfish such native brown trout in New Jersey streams. Actually, as chain pickerel, brook trout, pumpkinseed, brown trout are native to the British Isles and the redbreast sunfish, yellow perch, American shad, European mainland. What we catch here are wild American eels, white catfish and bullheads—the brown trout. majority of our native fishes are relatively un- Non-native fish such as brown trout and large- known. Some of the most interesting, rare and mouth bass reproduce in New Jersey waters, but important fishes are those native species that may that does not make them native.