PARTICIPANT INFORMATION SHEET Title of Project: Fractured Vision: Optometric Examination of Patients Following Stroke
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Heterotopias of Mental Health Care: the Role of Space in Experiences of Distress, Madness and Mental Health Service Use
Open Research Online The Open University’s repository of research publications and other research outputs Heterotopias of mental health care: The role of space in experiences of distress, madness and mental health service use. Thesis How to cite: Mcgrath, Laura (2012). Heterotopias of mental health care: The role of space in experiences of distress, madness and mental health service use. PhD thesis London South Bank University. For guidance on citations see FAQs. c 2012 The Author https://creativecommons.org/licenses/by-nc-nd/4.0/ Version: Version of Record Copyright and Moral Rights for the articles on this site are retained by the individual authors and/or other copyright owners. For more information on Open Research Online’s data policy on reuse of materials please consult the policies page. oro.open.ac.uk Heterotopias of mental health care: the role of space in experiences of distress, madness, and mental health service use. Laura McGrath A thesis submitted in partial fulfilment of the requirements of London South Bank University for the degree of Doctor of Philosophy 5th April 2012 1 Dedicated to Caroline McGrath 2 Acknowledgments Firstly, I am very grateful to all those who took the time to participate in this research and were willing to discuss their experiences with me. Two people in particular have been instrumental in the production of this thesis. First of all, I would like to express my gratitude to my supervisor, Paula Reavey, for her inspiring teaching in the area of mental health, as well as initially stirring my interest in space and materiality, and then for providing invaluable guidance, inspiration and friendship, over the course of this research. -

Cv Cantore.Pdf
Cristiano Cantore Research Hub E-mail: [email protected] Bank of England E-mail: [email protected] Threadneedle Street Web: http://www.cristianocantore.com London EC2R 8AH Phone: +44 (0)2034614469 Fields of Business Cycle Theory, Monetary and Fiscal Policy, Applied Macroeconomics Interest Current Research Advisor, Research Hub, Bank of England. 07/2021 to date Employment Reader in Economics (Part-Time), University of Surrey, Guildford. 08/2018 to date Other Centre for Macroeconomics (LSE), Euro Area Business Cycle Network, Central Bank Affiliations Research Association and Centre for International Macroeconomics (Surrey). Education Ph.D. in Economics University of Kent, (passed without corrections). 2011 Dissertation Examiners: Prof. M. Ellison (Oxford), Dr. K.Shibayama (Kent). M.Sc. in Economics, Pompeu Fabra University, Barcelona. 2005 B.A. in Economics and Statistics, L. Bocconi University, Milan. 2004 Previous Senior Research Economist, Research Hub, Bank of England. 09/2018 to 06/2021 Positions Senior Lecturer in Economics, University of Surrey, Guildford. 04/2013 to 07/2018 Visiting Professor, University of California San Diego. 02/2014 to 07/2014 Visiting Research Fellow, Banco de España, Madrid. 04/2012 to 08/2012 Lecturer in Economics, University of Surrey, Guildford. 09/2009 to 03/2013 Visiting Professor, University of Cagliari. 01/2012 to 02/2012 Intern, European Central Bank, Economics DG. 08/2008 to 10/2008 Intern, OECD, Economic Department, Country desk 1. 07/2007 to 09/2007 Publications (2021) Workers, Capitalists, and the Government: Fiscal Policy and Income (Re)Distribution, joint with Lukas Freund (University of Cambridge). Journal of Monetary Economics, 119, 58-74. -

G David Sands Sepnet Summer Placement
SEPnet Summer Placement Survey What software do students use on placements? How well do physics courses prepare them for using industry software? Veronica Benson SEPnet (South East Physics Network) Employer Liaison Director [email protected] Working together to promote excellence in Physics What is SEPnet? • The South East Physics Network (SEPnet) is a consortium of physics departments in 9 universities (Kent, Herts, OU, Portsmouth, Queen Mary, Royal Holloway, Southampton, Surrey & Sussex) • The SEPnet partners work together to deliver excellence in physics through collaboration, teaching and research • SEPnet includes: • an outreach programme to increase student interest in physics working with schools • A Graduate School (GRADnet) to develop technical and transferable skills of postgraduate research students • A collaborative research programme • An employer engagement programme to develop employability skills of undergraduates and postgraduate research students Working together to promote excellence in Physics What is the SEPnet Summer Placement Scheme? • An annual scheme offering placements to 2 nd and 3 rd (non final) year physics students at partner universities • Employers who recruit physics graduates and university supervisors offer 8-week summer projects • Projects are circulated to students who apply direct for roles • Students receive a £2,000 bursary funded by employers and SEPnet partner departments • SEPnet Employer Engagement Officers manage placements and visit students/supervisors during the placement • Students -
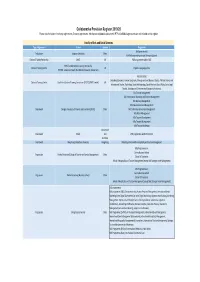
Register of Collaborative Provision
Collaborative Provision Register 2019/20 Please note that student exchange agreements, Erasmus agreements, Intentions to collaborate, placement / PTY or individual agreements are not included on this register Faculty of Arts and Social Sciences Type of Agreement Partner Country Programme BA Dance (level 6) Articulation Jiangnan University China MA Performance Practice and Research (Dance) Doctoral Training Partnership SeNSS UK PGR programmes within FASS AHRC funded Doctoral Training Partnership Doctoral Training Centre UK English & Languages, Arts TECHNE: London and South East Doctoral Research Consortium Social Sciences (including Economics, Human Geography, Management and Business Studies, Political Science and Doctoral Training Centre South East Doctoral Training Consortium (SE DTC) (ESRC funded) UK International Studies, Psychology, Social Anthropology, Social Work and Social Policy, Socio‐Legal Studies, Sociology and Environmental Energy and Resilience) BSc Tourism Management BSc International Hospitality and Tourism Management BSc Business Management MSc Financial Services Management Dual Award Dongbei University of Finance and Economics (DUFE) China MSc International Business Management MSc Retail Management MSc Tourism Development MSc Tourism Management MSc Tourism Marketing Amsterdam Dual Award ExSide and PGR programmes within Economics Germany Dual Award Hong Kong Polytechnic University Hong Kong PGR programmes within Hospitality and Tourism Management MSc Programmes in: Surrey Business School Progression Nankai University (College -

Procedure for the Investigation of Allegations of Misconduct in Research September 2013 Document Title
Procedure for the Investigation of Allegations of Misconduct in Research September 2013 Document title Procedure for the Investigation of Allegations of Misconduct in Research September 2013 Document author and department Responsible person and department Karen Musk, Research Manager Dr David Arrell, (Strategy and Policy), Pro Vice-Chancellor, Research and Innovation Services Directorate Approving body Date of approval Audit and Quality Committee 13 September 2013, Min 98 Academic Council 23 June 2015, Min 69 Review date Edition no. ID code September 2016 1 23 Review ongoing, please contact [email protected] if you have any queries. EITHER OR For public access online (internet)? For staff access only (intranet)? Tick as appropriate Tick as appropriate Yes 3 Yes For public access on request copy to be mailed Password protected Tick as appropriate Tick as appropriate Yes 3 No Yes No 3 External queries relating to the document to be referred in the first instance to the University Secretary: telephone +44 (0)23 9284 3195 or email [email protected] If you need this document in an alternative format, please contact +44 (0)23 9284 5776. The latest version of this document is always to be found at: http://policies.docstore.port.ac.uk/policy-023.pdf Contents Page no. Summary . 4 A. Introduction . 4 B. Scope . 5 C. Definitions . 5 D. Receipt of allegations . 7 E. Preliminary Investigation . 8 F. Preliminary Investigation: Findings and subsequent actions . 9 G. Formal Investigation . 10 H. Formal investigation: Findings and subsequent actions . 11 I. Reporting to the University Research and Enterprise and Innovation Committees . -

Oxford Brookes University
UK UNIVERSITY INTELLECTUAL PROPERTY RANKING 2020 Institution: Oxford Brookes University Location Of IP Policy: Click Here Ease Of Finding Document: Easy Current Tier: Tier 2 TIER 2 - CRITERIA A university-wide IP policy exists and is retrievable and downloadable, sometimes with a medium degree of difficulty, following a Google search using natural language and keyword combinations such as ‘UniName IP policy’ or ‘UniName intellectual property policy’. Some of the retrieved policies are unusually short (only 2 to 4 pages). Although the policy is exceptionally clear as to students’ IP ownership rights, it also includes IP policies for staff, academic visitors and other persons engaged with the university. Nonetheless, the students’ IP provisions of the IP policy may be viewed as a stand-alone section. OTHER UNIVERSITIES IN TIER 2 Imperial College London King’s College London University of Leeds University of Manchester University of London, Queen Mary Queen’s University Belfast University of Southampton University of York University of Aberdeen Heriot-Watt University University of Stirling Edinburgh Napier University Queen Margaret University University of the Highlands and Islands Abertay Univesity Ulster University The Open University Bangor University Aberystwyth University University of Arts London Aston University University of Bath Bath Spa University Birmingham City University Bishop Grosseteste University University of Bolton Bournemouth University Brunel University London Buckinghamshire New University University of Chichester University -

Uop Financial Review 2020 ACCESSIBLE FILE UPDATE
FINANCIAL REVIEW FOR THE YEAR ENDED 31 JULY 2020 Higher Education Corporation WELCOME FROM THE CHAIR OF GOVERNORS AND THE VICE-CHANCELLOR This has been a significant and memorable year for the University, and the Covid-19 pandemic has also made it a turbulent one both for the country and for the higher education sector. As these Financial Statements show, our sustained careful and prudent management of the University’s finances over many years will enable us to adapt and face the challenges ahead with greater certainty and confidence than might be the case elsewhere. Earlier in the year, we launched our exciting new University Strategy and Vision. By 2030, we will be the top modern university in the UK and one of the top 100 young universities in the world. That is our bold aspiration. Our Strategy is ambitious – and with good reason. We are a university that is focussed on real-world solutions and making a real difference to society. In 2017, we were awarded Gold in the Teaching Excellence Framework – the highest rating for teaching quality and in 2019 we were awarded £5.8 million by Research England to develop our plastics recycling research – the largest single research award in the University’s history. We are proud of our City and our heritage. Our outreach work with Portsmouth Football Club and Pompey in the Community, for example, brings the University into contact with many within our City and further afield who would otherwise not see education as an attainable pathway. Likewise, the recent pandemic has strengthened our links with our community as staff have volunteered to work in the “frontline” alongside other organisations to tackle the virus, our equipment and facilities have been utilised and loaned, and staff have designed and manufactured protective equipment to keep people safe. -

Geochemistry Group, Ggrip 2019 Programme and Abstracts
Geochemistry Group Research in Progress Meeting GGRiP 2019 University of Portsmouth 16-17th April 2019 Geochemistry Group, GGRiP 2019 Programme and Abstracts Dear Delegate, Welcome to the Geochemistry Group Research in Progress Meeting (GGRiP) 2019! The Group committee and the local organizers acknowledge our industrial partners: Sercon, OEA Labs, Metrohm, Cameca, Savillex, Teledyne, Agilent, SciMed, QMX and Thermo Scientific for their generous support of this meeting. We hope you enjoy your visit to Portsmouth. Craig Storey Local organiser, on behalf of the GG committee 2 Oral Presentations MONDAY 15TH APRIL Page Keynote/ Session Chair Start Authors Lead Affiliation Title no Student Arrival 17:00 ---- Public Lecture Craig Storey 1 17:30 Parrish, R University of Portsmouth Keynote Searching for the environmental smoking gun of Gulf War Illness Icebreaker Craig Storey - 18:30 - - -- TUESDAY 16TH APRIL Page Keynote/ Session Chair Start Authors Lead Affiliation Title no Student Arrival and Registration 09:00 - - -- Welcome & Intro Craig Storey - 09:45 - - -- 6 10:00 Badger, M.P.S. The Open University Keynote Fixing the alkenone palaeobarometer The Monterey Event and the Paleocene-Eocene Thermal Maximum - two 7 10:30 Babila, T.L. & Foster, G.L. University of Southampton contrasting oceanic carbonate system responses to LIP emplacement and Year of Carbon Inglis eruption Reconstructing the structure of Atlantic Ocean circulation during early-middle 8 10:45 McIntyre, A.J., Sexton, P.F. & Anand, P. The Open University Student Eocene extreme climatic warmth Coffee 11:00 Directly dating deformation with calcite U-Pb dating; the good, the bad and the 9 11:30 Mottram, C. University of Portsmouth Keynote ugly! Method/Technique Marie-Laure 10 12:00 Bevan, D.G., Coath, C.D., Lewis, J. -

This Item Was Submitted to Loughborough's
This item was submitted to Loughborough’s Institutional Repository by the author and is made available under the following Creative Commons Licence conditions. For the full text of this licence, please go to: http://creativecommons.org/licenses/by-nc-nd/2.5/ Email Training Significantly Reduces Email Defects Anthony Burgess*, Thomas Jackson and Janet Edwards Computer Science Department, Loughborough University, Leicestershire, UK Abstract Organisations are now becoming aware of the problems associated with email use and are keen to reduce these defects. These email defects relate to the ineffective way that email is used within organisations, and are not only limited to the volume of email that is sent and received, but also the quality of the email content. Email defects lead to inefficiencies within the workplace as employees spend more time dealing with email rather than doing other aspects of their job. This paper firstly examines how email is used within a large organisation and highlights the defects associated with email. The initial results show that these defects affect some groups of employees more than others. The paper also reports on the effectiveness of email training in reducing the defects associated with email use. The results show that some of these defects are related and that training can significantly reduce some of the email defects and improve the way people write emails. Key Words: Email, Electronic communication, Employee training, Employee productivity. *Corresponding author. Computer Science Department, Loughborough University, Loughborough, Leicestershire LE11 3TU, UK. Tel.: +44 (0)1509 228230; Fax: +44 (0)1509 211586. Email address: [email protected] (A. -

Coventry University Repository for the Virtual Environment (CURVE)
Coventry University Coventry University Repository for the Virtual Environment (CURVE) Copyright © and Moral Rights for this thesis are retained by the author and/ or other copyright owners. A copy can be downloaded for personal non-commercial research or study, without prior permission or charge. This thesis cannot be reproduced or quoted extensively from without first obtaining permission in writing from the copyright holder(s). The content must not be changed in any way or sold commercially in any format or medium without the formal permission of the copyright holders. A NUMBER OF ILLUSTRATIONS HAVE BEEN REMOVED FOR COPYRIGHT REASONS. CONSULT THE PRINTED VERSION When referring to this work, full bibliographic details must be given: Woolner, A. (2010) ‘Using interactive digital media to engage children on the autistic spectrum’ Coventry University, Unpublished PhD Thesis. Available in the CURVE Thesis Collection: February 2011 http://curve.coventry.ac.uk/open Using interactive digital media to engage children on the autistic spectrum Alex Woolner A thesis submitted in partial fulfillment of the University’s requirements for the Degree of Doctor of Philosophy Coventry University School of Art and Design July 2009 Abstract The incidence of autism is increasing in the U.K., with as many as 1% of children now thought to be affected by an autistic spectrum disorder (ASD). This research explores the potential of emerging interactive digital media to engage children affected by an ASD, and the development of design strategies for future professional work in this field. This is accomplished through a literature and state of the art review, and by working alongside families and professionals involved in the provision of care for children with an ASD. -

British School of Brussels Uk Universities Fair 2020
BRITISH SCHOOL OF BRUSSELS UK UNIVERSITIES FAIR 2020 20th October Times in Brussels Time 17.30 – 18.00 18.15 – 18.45 19.00 – 19.30 19.45 – 20.15 20.30 – 21.00 Your Future in Tech Royal Veterinary College Information University of Westminster Information Abertay University Information Session (Abertay University) Abertay University Information Session Session Session Applying for Competitive Courses in the UK Studying Engineering in the UK University of Bristol Information Session Engineering opportunities at the University (University of Bath) of Bristol University of Bristol Information Session (Imperial College London) UK-US Joint Degree: The University of Understanding the Multiple Mini Interview Andrews BA International Honours (MMI) Imperial College London Information Imperial College London Information programme University of St Andrews Information Royal Veterinary College Session Session (University of St Andrews) Session University of St Andrews Information Royal Veterinary College Information London School of Economics Information London School of Economics Information Arts University Bournemouth Information Session Session Session Session Session Falmouth University Information Session Falmouth University Information Session University of Sussex Information Session University of Sussex Information Session Newcastle University Information Session Choosing a degree to study in the UK Arts University Bournemouth Information Choosing the right fit UK university (University of Leeds) University of Leeds Information Session Session -

College Codes (Outside the United States)
COLLEGE CODES (OUTSIDE THE UNITED STATES) ACT CODE COLLEGE NAME COUNTRY 7143 ARGENTINA UNIV OF MANAGEMENT ARGENTINA 7139 NATIONAL UNIVERSITY OF ENTRE RIOS ARGENTINA 6694 NATIONAL UNIVERSITY OF TUCUMAN ARGENTINA 7205 TECHNICAL INST OF BUENOS AIRES ARGENTINA 6673 UNIVERSIDAD DE BELGRANO ARGENTINA 6000 BALLARAT COLLEGE OF ADVANCED EDUCATION AUSTRALIA 7271 BOND UNIVERSITY AUSTRALIA 7122 CENTRAL QUEENSLAND UNIVERSITY AUSTRALIA 7334 CHARLES STURT UNIVERSITY AUSTRALIA 6610 CURTIN UNIVERSITY EXCHANGE PROG AUSTRALIA 6600 CURTIN UNIVERSITY OF TECHNOLOGY AUSTRALIA 7038 DEAKIN UNIVERSITY AUSTRALIA 6863 EDITH COWAN UNIVERSITY AUSTRALIA 7090 GRIFFITH UNIVERSITY AUSTRALIA 6901 LA TROBE UNIVERSITY AUSTRALIA 6001 MACQUARIE UNIVERSITY AUSTRALIA 6497 MELBOURNE COLLEGE OF ADV EDUCATION AUSTRALIA 6832 MONASH UNIVERSITY AUSTRALIA 7281 PERTH INST OF BUSINESS & TECH AUSTRALIA 6002 QUEENSLAND INSTITUTE OF TECH AUSTRALIA 6341 ROYAL MELBOURNE INST TECH EXCHANGE PROG AUSTRALIA 6537 ROYAL MELBOURNE INSTITUTE OF TECHNOLOGY AUSTRALIA 6671 SWINBURNE INSTITUTE OF TECH AUSTRALIA 7296 THE UNIVERSITY OF MELBOURNE AUSTRALIA 7317 UNIV OF MELBOURNE EXCHANGE PROGRAM AUSTRALIA 7287 UNIV OF NEW SO WALES EXCHG PROG AUSTRALIA 6737 UNIV OF QUEENSLAND EXCHANGE PROGRAM AUSTRALIA 6756 UNIV OF SYDNEY EXCHANGE PROGRAM AUSTRALIA 7289 UNIV OF WESTERN AUSTRALIA EXCHG PRO AUSTRALIA 7332 UNIVERSITY OF ADELAIDE AUSTRALIA 7142 UNIVERSITY OF CANBERRA AUSTRALIA 7027 UNIVERSITY OF NEW SOUTH WALES AUSTRALIA 7276 UNIVERSITY OF NEWCASTLE AUSTRALIA 6331 UNIVERSITY OF QUEENSLAND AUSTRALIA 7265 UNIVERSITY