GORE™ STA-PURE™ Fluid Sampling System Is a Fully Disposable System for Biopharmaceutical Fluid Sampling
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

General Information
General Information: Summer dresses, made from lightweight fabric like silk organza or printed cotton, were popular during the time of the early bustle era from 1869-1876 for excursions to the sea or sporting activities like tennis. Hence the name “seaside costume” comes. Beside strong and light colors also striped fabrics were popular. Striped fabric often was cut on the bias for ruches and decorations to create lovely patterns. At the beginning of the era skirts were supported by smaller crinolines with an additional bustle at the back. At the middle of the seventies the crinoline was displaced by the actual tournure or “Cul de Paris”. Information’s about the sewing pattern: A seam allowance of 5/8” (1,5cm) is included, except other directions directly on the sewing pattern. Transfer all marks carefully when cutting the fabric. Pleas always do a mockup first. To get the desired shape the dress should be worn over a corset and suitable underpinnings. The dress is intended to be worn for more sporting activities, so it is designed to be worn over a small to a medium size bustle pad. If you want to wear the dress over a small crinoline or a larger bustle you have to spread the back width of the skirt to the hip era. Plan to make two or three pleats into the skirt gore #2 and #3 at the hip section. The front waist piece and the front apron are cut as one piece, at the side and the back the apron pieces are sewn on and folded into regular pleats at the back. -

Rando – Introducing the World's First Ski Suit in 3-Layer Gore-Tex® Active
Press release February 2013 Rando – introducing the world’s first ski suit in 3-layer Gore-Tex® Active Perhaps the secret of Randonée lies in the contrast of struggling up the mountain only to later enjoy the ride down. Perhaps it is the science of finding snow that no one else has broken before. Or perhaps just to enjoy the vast landscape. Regardless of the reason, the activity places special demands on the equipment. For the 2013 winter season Haglöfs is introducing a new extensive collection for skiers who want to explore far away from the lift system and pistes. All of these products, which are gathered under the RANDO family, were developed with a focus on safety and being able to quickly and easily adapt the clothing based on changing weather conditions or whether you are headed up or down the mountain. One of this year’s big releases is RANDO AS SUIT – the world’s first ski suit in 3-layer Gore-Tex® Active. During its product development stage, Haglöfs focused on taking advantage of the properties of Gore’s Active technology in order to be able to offer the lightest and the most comfortable, waterproof and breathable skiing garment on the market. RANDO AS SUIT has an unembellished design, but with all of the product features a skier would expect, such as a helmet-compatible, adjustable 3-way hood, a DWR-treated outer surface and pockets strategically placed so that they are accessible even when carrying a backpack. The suit also has long, reinforced and waterproof zippers at the armpits and along the thighs in order to maximize ventilation. -

Cotehardie Construction from Extant Pieces
Cotehardie Construction from Extant Pieces Mistress Mairghead de Chesholme [email protected] Mka Jacquelyne Aubuchon Uppsala gown reconstruction http://www.kostym.cz/Anglicky/1_Originaly/01_Goticke/I_01_13.htm Very few examples of clothing from the High Middle Ages have survived to the present day. Of those garments, even fewer are available for study to the amateur costumer. Marc Carlson’s website “Some Clothing of the Middle Ages” provides an invaluable resource for the costumer to find garments with which to make comparisons. For this examination, I have selected five garments for comparison. These garments include the Soderkoping kirtle, Herjolfnes No. 42, Herjolfnes No. 39, Herjolfnes No. 38, and the Uppsala gown. The Herjolfnes garments immediately precede the cotehardie in fashion, but are not necessarily cotes. What they show is a continuity of cut and style that can arguably be carried over to application in construction of cotehardies. For example, think of a man’s dress shirt. Envision the places where the seams lie on a modern shirt. One hundred years ago, the technology was radically different, but the seams will correspond on a man’s shirt from the 1800’s. Clothing construction is constantly tweaked, skirts get longer and shorter, dresses range from fitted to volumous, but the basic lines for seams remain the same. By utilizing what is known on the above five garments one can reasonably reproduce a cotehardie using a pattern that would have been plausible at the time. Soderkoping Kirtle http://personal.utulsa.edu/~marc-carlson/cloth/soder.html The first garment and most degraded of the five is the Soderkoping kirtle. -

Simplified Sewing: Hems
South Dakota State University Open PRAIRIE: Open Public Research Access Institutional Repository and Information Exchange SDSU Extension Fact Sheets SDSU Extension 1964 Simplified Sewing: Hems Cooperative Extension South Dakota State University Follow this and additional works at: https://openprairie.sdstate.edu/extension_fact Recommended Citation South Dakota State University, Cooperative Extension, "Simplified Sewing: Hems" (1964). SDSU Extension Fact Sheets. 865. https://openprairie.sdstate.edu/extension_fact/865 This Fact Sheet is brought to you for free and open access by the SDSU Extension at Open PRAIRIE: Open Public Research Access Institutional Repository and Information Exchange. It has been accepted for inclusion in SDSU Extension Fact Sheets by an authorized administrator of Open PRAIRIE: Open Public Research Access Institutional Repository and Information Exchange. For more information, please contact [email protected]. .. ... -~--- - --v · . --,-.-.- , -. ·. ..•, .. ·'··· .••,.,. - .t:\. --(. ......., • ... ·\•i• ....,.,•-•1•,··...- .,.. "\' ·,- • • • -:\. -: ·):.~\:,..,:}:,:.·,:,..,:;:,:.~:,-:-,:.0:.- ·,~\:,.!;;;; ··:,:.\!.t:,-;..:,t;.,:,:,:.i:1:,:.'.:.•; .-·F_:,~::::_:.~:~~~~~;.:::z~:;:'.·---~-~;:~~L ::·.1~~~~1::.:;.:_;~~: File Copy FS244 THE l-lE~I of your dress can have the "professional - Wearer should stand in a natural position with look" or the "homemade look." In general, the best arms at sides and weight on both feet. hem is the least conspicuous, and every step in making a hem is planned with this in mind There are several methods of marking a hem. You may use any one of these: The professional touch is recognize-cl by the follow- ing characteristics: Various types of chalk markers or pin markers - Inconspicuous from the right side Yardstick - Even distance from the floor Tailor's square. - Wide enough for good proportion and to gi\.re With some of these you can mark enough weight to hang well your own hem; others require a - Even in width help«. -

Dressmaking up to Date
PRICE, 25 CENTS or Is. PUBLISHED BY THE BUTTERICK PUBLISHING COMPANY, Limited AT THE BUTTERICK BUILDING, NEW YORK PARIS LONDON NEW YORK TORONTO Copyright, /QOj, by The Butter ick Publishing Co., Limited. Entered at Stationers’ Hall. A ll rights reserved. ■r o: ; < A Dressmaking, TUp to Date h.-K'ARV,^ rorS^BiS1 f'flsi $cp»es jits,wiYW( { . > i SfcP Hi ; « Ooiwngns umt J-^/ o2 &. I c/C61 / a 1X733! COPY f;s> . 3 FRONTISPIECE —THE SEWING CIRCLE HAND-SEWING STITCHES IMPORTANT POINTS AND AIDS IN DRESSMAKING THE CORRECT METHOD OF ALTERING PATTERNS SHIRT-BLOUSES DRAPED WAISTS SKIRTS NOVEL, ARTISTIC SEAMS WEDDING AND EVENING GOWNS THE TAILOR-MADE GOWN COATS AND JACKETS PRACTICAL AND ORNAMENTAL STITCHES BIAS BANDS AND FOLDS—TURNING CORNERS AN EMPIRE TEA-GOWN DESIRABLE GARMENTS FOR MATERNITY WEAR MAKING /ND FINISHING UNDERWEAR THE BATH-ROBE. CHILDREN’S CLOTHES BOYS’ SUITS XTlp to 5>ate inning (EirrU SDressmahtrtg, TUp to Date SIMPLE SEWING STITCE1ES AKING A KNOT.—Holding the threaded needle in the right hand, twist the end of the thread once and a half, around the forefinger of the left hand; press, roll downward on the ball of the thumb, twisting once or twice; slip off and draw down M with the middle finger of the left hand. BASTING.—There are two kinds of basting; even and uneven. In even basting the stitches; and spaces are the same length; in uneven basting, as its name implies, the stitches are so formed that they are not of equal length. EVEN BASTING STITCH.—Start with a knot in basting and always have it on the right side; it is more easily removed. -

Style Set 2 for Garment Designer
Style Set 2 for Garment Designer Welcome to Style Set 2. This set provides you with 50+ additional style elements that will mix and match with the original Garment Designer & Style Set 1 styles . Key to Style Information/Illustrations Red Lines/Curves: Show you new points involved in the style, plus the segments (either straight or curved) that exist with the style. Use this information to assist you in choosing styles for custom design ideas. Black/Red Lines: Show you the pattern built through the combination of style menus. Combination: The style menu options chosen for the style shown. Use these as inspiration. Illustration: Further inspiration. Comments: Helpful information about the style. Thanks to Jon Balcalski, Mesa College (San Diego) student, who assisted with the illustrations as part of his Honors Computer Fashion course. Style Set 2 © 2002, All Rights Reserved Susan Lazear, Cochenille Design Studio Encinitas, CA 92023-4276 www.cochenille.com [email protected] 1 Installation: Double-click on the StyleSet2Installer file found on the CD you have received with this package. The installa- tion will happen automatically. Various files will be updated in your Garment Designer folder. Note: 1. The Style Set 2 installation will update your Garment Designer application file. It will also update your Style Set 1 (SS1) file, should you have SS1 installed. 2. It is highly recommended that you only have one copy of Garment Designer on your computer. This will avoid confusing the Installer. 3. With the millions of combinations now available in Garment Designer (with Style Set 1 and Style Set 2), it is likely that there is a style combination that simply cannot work, or doesn’t work properly. -
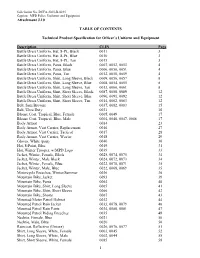
Attachment J.10 1 TABLE of CONTENTS Technical Product
Solicitation No. DCFA-2010-B-0235 Caption: MPD Police Uniforms and Equipment Attachment J.10 TABLE OF CONTENTS Technical Product Specification for Officer’s Uniform and Equipment Description CLIN Page Battle Dress Uniform, Hat, 8-Pt., Black 0011 3 Battle Dress Uniform, Hat, 8-Pt., Blue 0010 3 Battle Dress Uniform, Hat, 8-Pt., Tan 0015 3 Battle Dress Uniform, Pants, Black 0007, 0052, 0053 4 Battle Dress Uniform, Pants, Blue 0006, 0050, 0051 4 Battle Dress Uniform, Pants, Tan 0012, 0058, 0059 4 Battle Dress Uniform, Shirt, Long Sleeve, Black 0009, 0056, 0057 8 Battle Dress Uniform, Shirt, Long Sleeve, Blue 0008, 0054, 0055 8 Battle Dress Uniform, Shirt, Long Sleeve, Tan 0013, 0060, 0061 8 Battle Dress Uniform, Shirt, Short Sleeve, Black 0087, 0088, 0089 12 Battle Dress Uniform, Shirt, Short Sleeve, Blue 0090, 0091, 0092 12 Battle Dress Uniform, Shirt, Short Sleeve, Tan 0014, 0062, 0063 12 Belt, Sam Browne 0037, 0082, 0083 15 Belt, Ultra Duty 0031 16 Blouse Coat, Tropical, Blue, Female 0005, 0049 17 Blouse Coat, Tropical, Blue, Male 0004, 0046, 0047, 0048 17 Body Armor 0084 23 Body Armor, Vest Carrier, Replacement 0016 27 Body Armor, Vest Carrier, Tactical 0017 28 Body Armor, Vest Carrier, Wavier 0018 29 Gloves, White (pair) 0040 30 Hat, 8-Point, Blue 0019 31 Hat, Winter Trooper, w/MPD Logo 0039 33 Jacket, Winter, Female, Black 0025, 0074, 0075 34 Jacket, Winter, Male Black 0024, 0072, 0073 34 Jacket, Winter, Female, Blue 0023, 0070, 0071 35 Jacket, Winter, Male, Blue 0022, 0068, 0069 35 Motorcycle Breeches, Winter/Summer 0036 36 Mountain -

Fabric Manipulation and Its Impact on Fashion Designs Education (Part 1)
IOSR Journal of Research & Method in Education (IOSR-JRME) e-ISSN: 2320–1959.p- ISSN: 2320–1940 Volume 9, Issue 5 Ser. I. (Sep. - Oct .2019), PP 43-52 www.iosrjournals.org Fabric Manipulation and its impact on Fashion Designs Education (part 1) Nashwa El Shafei. PhD1, 2Laila Al Maghrabi. PhD Ready-made garment Technology'sReadymade Garment 1Faculty of Applied Arts Damietta University- 5th Assembly 2Higher Institute of Applied Arts Corresponding Author: Nashwa El Shafei PhD Abstract: The present research demonstrates an interaction technique as fabric implementations for putting garments on a three-dimensional character with manipulating them with its application on fashion. Furthermore to realize and examine the potential of the fabrics and materials which could be manipulated using different techniques and processes to innovative designs and artwork which could be applied to fine art, originate textile or fashion scenario. On the other hand, to develop different ways of altering fabric to provide contrasts, to create a sense of fullness, and create surface effects. Some of these methods are very old, but contemporary fabric artists and fashion designers continued to use them and adapt them in new styles. Key words: Fabric implementations,fullness,surface effects, contemporary fabric. ----------------------------------------------------------------------------------------------------------------------------- ---------- Date of Submission: 27-08-2019 Date of Acceptance: 11-09-2019 ----------------------------------------------------------------------------------------------------------------------------- -

Special MARKETS Spring 2012
SPECIAL MARKETS SPRING 2012 MEn’S Pureformance™ Polo with Contrast Trim A fresh and updated alternative to our Exacting standards and unequaled Royalty Pureformance™ solid lisle. Mercerized Cotton for exceptional luster, color fastness craftsmanship and stability, combined with polyester yarn for moisture wicking technology. Features a fashionable striped fabric neck lining, contrast color neck tape, and a Fairway & Greene, founded in 1995, is handsome chambray fabric lining in the widely acknowledged as the leader in the White neck placket. Constructed with the Fairway premium green grass and resort markets. With & Greene signature collar, open sleeves, and an unparalleled emphasis on product quality, a three-button placket with cross stitched customer service, and sales support. buttons. Fairway & Greene continues to grow its product assortment as it builds its image on and off the course through markets in Corporate America, Secretariat PGA Tour events, Colleges and Resorts. C31120C Fairway & Greene responds by ensuring the COLORS highest quality product, superior customer Royalty • White • Secretariat • Racing Red service, and on-time delivery. SIZES SUGGESTED RETAIL SM-3XL $80.00 Through exacting standards and unequaled craftsmanship, Fairway & Greene offers a Racing Red complete collection of premium products. Whether it is the “signature”, performance, or F&G Tech collections of knits, clubhouse wovens, luxurious merino wool sweaters, or active cashmere, each item is made with the highest standards. F&G’s attention to detail, with a classic and stylized comfort approach, is recognized throughout its loyal customer base. 2 FAIrwaY & GREENE LADIES Classic Stretch Pique Shirt A stylish ladies silhouette made from Mercerized Cotton and 5% Lycra® for added stretch and comfort. -

Shion Design, Textile Technology, Textile Management & Fashion Communication
Textile Craft, Textile and Fashion Design, Textile Technology, Textile Management & Fashion Communication Special Edition: Sustainability & Innovation in the Fashion Field The Nordic Textile Journal is a peer reviewed international journal publishing high-quality articles dedicated to research and artistic development related to textiles and fashion. The Journal considers only manuscripts that have not been published elsewhere. The Journal is published in English by the CTF Centre of Textile Research in Borås, Sweden. Copyright is by The Textile Research Centre, CTF the publisher and the authors. The Swedish School of Textiles University of Borås The Journal is issued in printed form, normally one issue annually. SE-501 90 BORÅS Articles from earlier issues will also be available in electronic form SWEDEN via BADA, the Borås Academic Digital Archive http://bada.hb.se/ Tel: +46 33 435 43 93 handle/2320/1556 Fax: +46 33 435 40 09 E-mail [email protected] Manuscripts in the following categories will be considered for Web: www.hb.se/ths/ctf publication: Publisher 1. Research articles: A research article is a regular article which CTF Publishing aims to present new findings in textile and fashion research. Executive Editor 2. Articles on artistic development: An article on artistic Håkan Torstensson development aims to present artistic progress and may relate to practice-based design research, design Assistant Executive Editor methodology or novel achievements in textile art and craft. Katrin Tijburg 3. Notes: A note is a short article, which aims to report Edited by new findings. Karin M Ekström Agneta Nordlund Andersson 4. Review articles: A review article aims to present already Katrin Tijburg existing findings and may be a book review or an Håkan Torstensson exhibition review. -

Globe Guard™ System
INTRODUCING ™ GLOBE GUARD EXPOSURE REDUCTION SYSTEM GLOBE. ATHLETIC GEAR FOR FIREFIGHTERS.TM PROTECTING FIREFIGHTERS, NOW AND IN THE FUTURE. The Globe Guard™ System, in combination with the most innovative turnout gear from Globe, provides firefighters the gold standard in helping reduce exposure to toxic substances without impeding performance. Globe and MSA are committed to delivering premium performance solutions and supporting scientific research to help address long-term firefighter health and safety. Continually improving turnout gear specifications is one of the many steps necessary for departments to help reduce exposure to toxic substances for their members. GLOBE GUARD™ JACKET COMPONENT GLOBE GUARD™ PANT FLY COMPONENT Sewn into the thermal liner Constructed of two layers above the hemline and – GORE® CROSSTECH® constructed of two layers black moisture barrier – GORE® CROSSTECH® and TECASAFE® PLUS for black moisture barrier moisture management, high and TECASAFE® PLUS for breathability, flame resistance, moisture management, and active moisture wicking – high breathability, flame and installed at the base of the resistance, and active moisture wicking – to help reduce exposure at fly to help reduce exposure. the jacket and pants interface. The Globe Guard™ Jacket Component is automatically engaged as part of the liner system when the jacket closures are secured. GLOBE GUARD™ PANT CUFF COMPONENT OTHER CONSIDERATIONS Constructed of two layers On the jacket, a front closure with a zipper inside and hook and loop – GORE® CROSSTECH® outside is recommended to help reduce exposure when properly black moisture barrier and secured. On the pants, the addition of the inside gusset helps to reduce TECASAFE® PLUS – and sewn exposure when the hook and loop fly closure is properly closed. -

Report Resumcs
I REPORT RESUMCS ED 0!? 782 VT 001 07.:: PATTERNMAKING AND DESIGN. BY- COLICCHIO, ANTOINETTE J. RUTGERS, THE STATE UNIV., NEW BRUNSWICK, N.J. NEW JERSEY STATE DEPT. OF EDUCATION, TRENTON PUB DATE JAN 67 EDRS PRICE ME-$1.25 HC-$12.52 313P. DESCRIPTORS- *STUDY GUIDES, *TRADE AND INDUSTRIAL EDUCATION, *CLOTHING DESIGN, #PATTERNMAKING, HIGH SCHOOLS, NEEDLE TRADES, NEW BRUNSWICK, TRENTON SKILLS IN PATTERNMAKING ARE IMPOP'ANT TO ALL EMPLOYE-9 IN nIC DESIGNING, ROOM TM !P -we-stop rAcurwr mAMIMACTURE. THE OBJECTIVE OF THIS COURSE OF STUDY IS TO ACQUAINT THE STUDENT vii_im THE TOOLS AND SYMBOLS OF PATTENMAKING AND TO HELP HIM MASTER THE DASiC FUNDAMENTALS OF PATTERN DEVELOPMENT. II FOLLOWS THE COURSE OF STUDY APPROVED BY THE BOARD OF EDUCATION AND WAS TESTED IN VARIOUS CLASSROOMS. THEORY AND PRINCIPLES ARE COMBINED WITH PRACTICAL AND CREATIVE APPLICATION IN COSTUME DESIGN. UNITS ARC--(1) INTRODUCTION TO PATTERNMAKING AND DESIGN,(2) SLOPERS,(3) WAISTS, (4) NECKLINES,(5) CLOSINGS AND EXTENSIONS,(6) COLLARS, (7) SLEEVES,(8) SKIRTS,(9) POCKETS] AND (10) BELTS. EACH UNIT INCLUDES OBJECTIVES, RELATED INFORMATION, ASSIGNMENTS, AND ILLUSTRATIONS. THE COURSE REQUIRES THREE PERIODS PER WEEK FOR 1 1/2 YEARS. STUDENTS SHOULD BE OF HIGH SCHOOL AGE WITH APTITUDES AND GOALS FOR THE NEEDLE TRADES. THE TEACHER SHOULD BE A NEEDLE TRADES INSTRUCTOR. INCLUDED ARE OBJECTIVE AND PERFORMANCE TESTS, ILLUSTRATIONS, AND A BIBLIOGRAFWi. THIS DOCUMENT IS ALSO .A.VAILAIXE FROM THE VOCATIONAL-TECHNICAL CURRICULUM LAt*RATORY, RUTGERS UNIVERSITY, 10 SEMINARY PLACE, NEW BRUNSWICK, NEW JERSEY 08903, FOR $2.00. (MS) .. Z.41040 41A.:.; vp 4 40 004040 41 400 Er .