Literature Review and Data Availability for Splendid Alfonsino Stock Assessment
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Order BERYCIFORMES ANOPLOGASTRIDAE Fangtooths (Ogrefish) by J.A
click for previous page 1178 Bony Fishes Order BERYCIFORMES ANOPLOGASTRIDAE Fangtooths (ogrefish) by J.A. Moore, Florida Atlantic University, USA iagnostic characters: Small (to about 160 mm standard length) beryciform fishes.Body short, deep, and Dcompressed, tapering to narrow peduncle. Head large (1/3 standard length). Eye smaller than snout length in adults, but larger than snout length in juveniles. Mouth very large and oblique, jaws extend be- hind eye in adults; 1 supramaxilla. Bands of villiform teeth in juveniles are replaced with large fangs on dentary and premaxilla in adults; vomer and palatines toothless. Deep sensory canals separated by ser- rated ridges; very large parietal and preopercular spines in juveniles of one species, all disappearing with age. Gill rakers as clusters of teeth on gill arch in adults (lath-like in juveniles). No true fin spines; single, long-based dorsal fin with 16 to 20 rays; anal fin very short-based with 7 to 9 soft rays; caudal fin emarginate; pectoral fins with 13 to 16 soft rays; pelvic fins with 7 soft rays. Scales small, non-overlapping, spinose, goblet-shaped in adults; lateral line an open groove partially bridged by scales; no enlarged ventral keel scutes. Colour: entirely dark brown or black in adults. Habitat, biology, and fisheries: Meso- to bathypelagic, at depths of 75 to 5 000 m. Carnivores, with juveniles feeding on mainly crustaceans and adults mainly on fishes. May sometimes swim in small groups. Uncommon deep-sea fishes of no commercial importance. Remarks: The family was revised recently by Kotlyar (1986) and contains 1 genus with 2 species throughout the tropical and temperate latitudes. -

New Insights on the Sister Lineage of Percomorph Fishes with an Anchored Hybrid Enrichment Dataset
Molecular Phylogenetics and Evolution 110 (2017) 27–38 Contents lists available at ScienceDirect Molecular Phylogenetics and Evolution journal homepage: www.elsevier.com/locate/ympev New insights on the sister lineage of percomorph fishes with an anchored hybrid enrichment dataset ⇑ Alex Dornburg a, , Jeffrey P. Townsend b,c,d, Willa Brooks a, Elizabeth Spriggs b, Ron I. Eytan e, Jon A. Moore f,g, Peter C. Wainwright h, Alan Lemmon i, Emily Moriarty Lemmon j, Thomas J. Near b,k a North Carolina Museum of Natural Sciences, Raleigh, NC, USA b Department of Ecology & Evolutionary Biology and Peabody Museum of Natural History, Yale University, New Haven, CT 06520, USA c Program in Computational Biology and Bioinformatics, Yale University, New Haven, CT 06520, USA d Department of Biostatistics, Yale University, New Haven, CT 06510, USA e Marine Biology Department, Texas A&M University at Galveston, Galveston, TX 77554, USA f Florida Atlantic University, Wilkes Honors College, Jupiter, FL 33458, USA g Florida Atlantic University, Harbor Branch Oceanographic Institution, Fort Pierce, FL 34946, USA h Department of Evolution & Ecology, University of California, Davis, CA 95616, USA i Department of Scientific Computing, Florida State University, 400 Dirac Science Library, Tallahassee, FL 32306, USA j Department of Biological Science, Florida State University, 319 Stadium Drive, Tallahassee, FL 32306, USA k Peabody Museum of Natural History, Yale University, New Haven, CT 06520, USA article info abstract Article history: Percomorph fishes represent over 17,100 species, including several model organisms and species of eco- Received 12 April 2016 nomic importance. Despite continuous advances in the resolution of the percomorph Tree of Life, resolu- Revised 22 February 2017 tion of the sister lineage to Percomorpha remains inconsistent but restricted to a small number of Accepted 25 February 2017 candidate lineages. -

Jolanta KEMPTER*, Maciej KIEŁPIŃSKI, Remigiusz PANICZ, and Sławomir KESZKA
ACTA ICHTHYOLOGICA ET PISCATORIA (2016) 46 (4): 287–291 DOI: 10.3750/AIP2016.46.4.02 MICROSATELLITE DNA-BASED GENETIC TRACEABILITY OF TWO POPULATIONS OF SPLENDID ALFONSINO, BERYX SPLENDENS (ACTINOPTERYGII: BERYCIFORMES: BERYCIDAE)—PROJECT CELFISH—PART 2 Jolanta KEMPTER*, Maciej KIEŁPIŃSKI, Remigiusz PANICZ, and Sławomir KESZKA Division of Aquaculture, West Pomeranian University of Technology, Szczecin, Kazimierza Krolewicza 4, 71-550 Szczecin, Poland Kempter J., Kiełpinski M., Panicz R., Keszka S. 2016. Microsatellite DNA-based genetic traceability of two populations of splendid alfonsino, Beryx splendens (Actinopterygii: Beryciformes: Berycidae)— Project CELFISH—Part 2. Acta Ichthyol. Piscat. 46 (4): 287–291. Background. The study is a contribution to Project CELFISH which involves genetic identifi cation of populations of fi sh species presenting a particular economic importance or having a potential to be used in the so-called commercial substitutions. The EU fi sh trade has been showing a distinct trend of more and more fi sh species previously unknown to consumers being placed on the market. Molecular assays have become the only way with which to verify the reliability of exporters. This paper is aimed at pinpointing genetic markers with which to label and differentiate between two populations of splendid alfonsino, Beryx splendens Lowe, 1834, a species highly attractive to consumers in Asia and Oceania due to the meat taste and low fat content. Material and methods. DNA was isolated from fragments of fi ns collected at local markets in Japan (MJ) (n = 10) and New Zealand (MNZ) (n = 18). The rhodopsin gene (RH1) fragment and 16 microsatellite DNA fragments (SSR) were analysed in all the individuals. -

Order BERYCIFORMES ANOPLOGASTRIDAE Anoplogaster
click for previous page 2210 Bony Fishes Order BERYCIFORMES ANOPLOGASTRIDAE Fangtooths by J.R. Paxton iagnostic characters: Small (to 16 cm) Dberyciform fishes, body short, deep, and compressed. Head large, steep; deep mu- cous cavities on top of head separated by serrated crests; very large temporal and pre- opercular spines and smaller orbital (frontal) spine in juveniles of one species, all disap- pearing with age. Eyes smaller than snout length in adults (but larger than snout length in juveniles). Mouth very large, jaws extending far behind eye in adults; one supramaxilla. Teeth as large fangs in pre- maxilla and dentary; vomer and palatine toothless. Gill rakers as gill teeth in adults (elongate, lath-like in juveniles). No fin spines; dorsal fin long based, roughly in middle of body, with 16 to 20 rays; anal fin short-based, far posterior, with 7 to 9 rays; pelvic fin abdominal in juveniles, becoming subthoracic with age, with 7 rays; pectoral fin with 13 to 16 rays. Scales small, non-overlap- ping, spinose, cup-shaped in adults; lateral line an open groove partly covered by scales. No light organs. Total vertebrae 25 to 28. Colour: brown-black in adults. Habitat, biology, and fisheries: Meso- and bathypelagic. Distinctive caulolepis juvenile stage, with greatly enlarged head spines in one species. Feeding mode as carnivores on crustaceans as juveniles and on fishes as adults. Rare deepsea fishes of no commercial importance. Remarks: One genus with 2 species throughout the world ocean in tropical and temperate latitudes. The family was revised by Kotlyar (1986). Similar families occurring in the area Diretmidae: No fangs, jaw teeth small, in bands; anal fin with 18 to 24 rays. -
![Stock Status Report: Alfonsino [BYS] DOC/SC/11/2019 South East](https://docslib.b-cdn.net/cover/1518/stock-status-report-alfonsino-bys-doc-sc-11-2019-south-east-341518.webp)
Stock Status Report: Alfonsino [BYS] DOC/SC/11/2019 South East
Stock Status Report: Alfonsino [BYS] DOC/SC/11/2019 STATUS REPORT Beryx splendens Alfonsino FAO -ASFIS code: BYS 2019 Updated 21 November 2019 South East Atlantic Fisheries Organization [SEAFO] 1 Stock Status Report: Alfonsino [BYS] DOC/SC/11/2019 TABLE OF CONTENTS 1. Description of the fishery ....................................................................................................................... 3 1.1 Description of fishing vessels and fishing gear .......................................................................... 3 1.2 Spatial and temporal distribution of fishing ............................................................................... 6 1.3 Reported retained catches and discards ..................................................................................... 9 1.4 IUU catch ............................................................................................................................... 12 2. Stock distribution and identity ............................................................................................................. 12 3. Data available for assessments, life history parameters and other population information ..................... 12 3.1 Fisheries and surveys data ....................................................................................................... 12 3.2 Length data and frequency distribution ................................................................................... 13 3.3 Length-weight relationships ................................................................................................... -

How Much Longer Will It Take?
How much longer will it take? A ten-year review of the implementation of United Nations General Assembly resolutions 61/105, 64/72 and 66/68 on the management of bottom fisheries in areas beyond national jurisdiction FULL REPORT – AUGUST 2016 DAVID SHALE/NATURE PICTURE LIBRARAY SHALE/NATURE DAVID Leiopathes sp., a deepwater black coral, has lifespans in excess of 4,200 years (Roark et al., 2009*), making it one of the oldest living organism on Earth. Specimen was located off the coast of Oahu, Hawaii, in ~400 m water depth. * Roark, E.B., Guilderson, T.P., Dunbar, R.B., Fallon, S.J., and Mucciarone, D.A., 2009. Extreme longevity in proteinaceous deep-sea corals. Proceedings of the National Academy of Sciences, 106: 520– 5208, doi: 10.1073/pnas.0810875106. © HAWAII UNDERSEA RESEARCH LABORATORY, TERRY KERBY AND MAXIMILIAN CREMER Contents Executive summary 03 1.0 Introduction 09 2.0 North Atlantic 10 2.1 Northeast Atlantic 10 2..2 Northwest Atlantic 21 3.0 South Atlantic 33 3.1 Southeast Atlantic 33 3.2 Southwest Atlantic and other non-RFMO areas 39 4.0 North Pacific 42 Citation: 5.0 South Pacific 49 Gianni, M., Fuller, S.D., Currie, D.E.J., Schleit, 6.0 Indian Ocean 60 K., Goldsworthy, L., Pike, B., Weeber, B., 7.0 Southern Ocean 66 Owen, S., Friedman, A. How much longer will it take? A ten-year 8.0. Mediterranean Sea 71 review of the implementation of United Annex 1. Acronyms 73 Nations General Assembly resolutions Annex 2. History of the UNGA negotiations 73 61/105, 64/72 and 66/68 on the management Annex 3. -

Updated Checklist of Marine Fishes (Chordata: Craniata) from Portugal and the Proposed Extension of the Portuguese Continental Shelf
European Journal of Taxonomy 73: 1-73 ISSN 2118-9773 http://dx.doi.org/10.5852/ejt.2014.73 www.europeanjournaloftaxonomy.eu 2014 · Carneiro M. et al. This work is licensed under a Creative Commons Attribution 3.0 License. Monograph urn:lsid:zoobank.org:pub:9A5F217D-8E7B-448A-9CAB-2CCC9CC6F857 Updated checklist of marine fishes (Chordata: Craniata) from Portugal and the proposed extension of the Portuguese continental shelf Miguel CARNEIRO1,5, Rogélia MARTINS2,6, Monica LANDI*,3,7 & Filipe O. COSTA4,8 1,2 DIV-RP (Modelling and Management Fishery Resources Division), Instituto Português do Mar e da Atmosfera, Av. Brasilia 1449-006 Lisboa, Portugal. E-mail: [email protected], [email protected] 3,4 CBMA (Centre of Molecular and Environmental Biology), Department of Biology, University of Minho, Campus de Gualtar, 4710-057 Braga, Portugal. E-mail: [email protected], [email protected] * corresponding author: [email protected] 5 urn:lsid:zoobank.org:author:90A98A50-327E-4648-9DCE-75709C7A2472 6 urn:lsid:zoobank.org:author:1EB6DE00-9E91-407C-B7C4-34F31F29FD88 7 urn:lsid:zoobank.org:author:6D3AC760-77F2-4CFA-B5C7-665CB07F4CEB 8 urn:lsid:zoobank.org:author:48E53CF3-71C8-403C-BECD-10B20B3C15B4 Abstract. The study of the Portuguese marine ichthyofauna has a long historical tradition, rooted back in the 18th Century. Here we present an annotated checklist of the marine fishes from Portuguese waters, including the area encompassed by the proposed extension of the Portuguese continental shelf and the Economic Exclusive Zone (EEZ). The list is based on historical literature records and taxon occurrence data obtained from natural history collections, together with new revisions and occurrences. -

Appendices Appendices
APPENDICES APPENDICES APPENDIX 1 – PUBLICATIONS SCIENTIFIC PAPERS Aidoo EN, Ute Mueller U, Hyndes GA, and Ryan Braccini M. 2015. Is a global quantitative KL. 2016. The effects of measurement uncertainty assessment of shark populations warranted? on spatial characterisation of recreational fishing Fisheries, 40: 492–501. catch rates. Fisheries Research 181: 1–13. Braccini M. 2016. Experts have different Andrews KR, Williams AJ, Fernandez-Silva I, perceptions of the management and conservation Newman SJ, Copus JM, Wakefield CB, Randall JE, status of sharks. Annals of Marine Biology and and Bowen BW. 2016. Phylogeny of deepwater Research 3: 1012. snappers (Genus Etelis) reveals a cryptic species pair in the Indo-Pacific and Pleistocene invasion of Braccini M, Aires-da-Silva A, and Taylor I. 2016. the Atlantic. Molecular Phylogenetics and Incorporating movement in the modelling of shark Evolution 100: 361-371. and ray population dynamics: approaches and management implications. Reviews in Fish Biology Bellchambers LM, Gaughan D, Wise B, Jackson G, and Fisheries 26: 13–24. and Fletcher WJ. 2016. Adopting Marine Stewardship Council certification of Western Caputi N, de Lestang S, Reid C, Hesp A, and How J. Australian fisheries at a jurisdictional level: the 2015. Maximum economic yield of the western benefits and challenges. Fisheries Research 183: rock lobster fishery of Western Australia after 609-616. moving from effort to quota control. Marine Policy, 51: 452-464. Bellchambers LM, Fisher EA, Harry AV, and Travaille KL. 2016. Identifying potential risks for Charles A, Westlund L, Bartley DM, Fletcher WJ, Marine Stewardship Council assessment and Garcia S, Govan H, and Sanders J. -

Fish Otoliths from the Late Maastrichtian Kemp Clay (Texas, Usa)
Rivista Italiana di Paleontologia e Stratigrafia (Research in Paleontology and Stratigraphy) vol. 126(2): 395-446. July 2020 FISH OTOLITHS FROM THE LATE MAASTRICHTIAN KEMP CLAY (TEXAS, USA) AND THE EARLY DANIAN CLAYTON FORMATION (ARKANSAS, USA) AND AN ASSESSMENT OF EXTINCTION AND SURVIVAL OF TELEOST LINEAGES ACROSS THE K-PG BOUNDARY BASED ON OTOLITHS WERNER SCHWARZHANS*1 & GARY L. STRINGER2 1Natural History Museum of Denmark, Zoological Museum, Universitetsparken 15, DK-2100, Copenhagen, Denmark; and Ahrensburger Weg 103, D-22359 Hamburg, Germany, [email protected] 2 Museum of Natural History, University of Louisiana at Monroe, Monroe, Louisiana 71209, USA, [email protected] *Corresponding author To cite this article: Schwarzhans W. & Stringer G.L. (2020) - Fish otoliths from the late Maastrichtian Kemp Clay (Texas, USA) and the early Danian Clayton Formation (Arkansas, USA) and an assessment of extinction and survival of teleost lineages across the K-Pg boundary based on otoliths. Riv. It. Paleontol. Strat., 126(2): 395-446. Keywords: K-Pg boundary event; Gadiformes; Heterenchelyidae; otolith; extinction; survival. Abstract. Otolith assemblages have rarely been studied across the K-Pg boundary. The late Maastrichtian Kemp Clay of northeastern Texas and the Fox Hills Formation of North Dakota, and the early Danian Clayton Formation of Arkansas therefore offer new insights into how teleost fishes managed across the K-Pg boundary as reconstructed from their otoliths. The Kemp Clay contains 25 species, with 6 new species and 2 in open nomenclature and the Fox Hills Formation contains 4 species including 1 new species. The two otolith associations constitute the Western Interior Seaway (WIS) community. -

Modelling the Distribution of Alfonsino, Beryx Splendens, Over The
Abstract.- Commercial and Modelling the distribution of scientificbottom longline catches of alfonsino, Beryx splendens, from alfonsino, Beryx splendens, over seamounts off New Caledonia were sampled to study length-frequency distributions. A total of 14,674 fish the seamounts of New Caledonia were measured. CPUE of Beryx splendens on two seamounts is mod- Patrick Lehodey elled in terms of length and depth. The data show that mean length in- Paul Marchal creases with depth; this is well de- scribed by a bivariate normal model Rene Grandperrin that estimates catch for a given sea- Centre ORSTOM, BP A5, Noumea, New Caledonia mount. In addition, the data show I that mean length also varies with the depth of the top of seamounts; this is described by a recursive model that is designed to predict ap- proximate catch for any seamount. A bottom longline fishery operated recursive model predicts catch on The limitations of both models are on the seamounts of the Exclusive any seamount. discussed, particularly with regard Economic Zone (EEZ) of New Cale- to temporal variation. donia from February 1988 to July 1991.l Three vessels were involved Material and methods but only one vessel was operated at any given time. The fishing effort, Data which totalled 4,691,635 hooks, fo- Alfonsino were captured with long- cused on five seamounts (B, C, D, line gear (Fig. 2). The main line, J, and K) whose summits are lo- averaging 4,000 m, was held on the cated at depths ranging from 500 1). bottom by means of terminal an- to 750 m (Fig. -
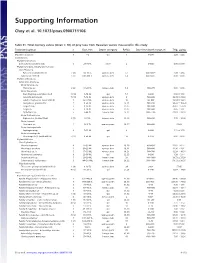
Supporting Information
Supporting Information Choy et al. 10.1073/pnas.0900711106 Table S1. Total mercury values (mean ؎ SD) of prey taxa from Hawaiian waters measured in this study Taxonomic group n Size, mm Depth category Ref(s). Day-time depth range, m THg, g/kg Mixed Zooplankton 5 1–2 epi 1 0–200 2.26 Ϯ 3.23 Invertebrates Phylum Ctenophora Ctenophores (unidentified) 3 20–30 TL other 2 0–600 0.00 Ϯ 0.00 Phylum Chordata, Subphylum Tunicata Class Thaliacea Pyrosomes (unidentified) 2 (8) 14–36 TL upmeso.dvm 2, 3 400–600ϩ 3.49 Ϯ 4.94 Salps (unidentified) 3 (7) 200–400 TL upmeso.dvm 2, 4 400–600ϩ 0.00 Ϯ 0.00 Phylum Arthropoda Subphylum Crustacea Order Amphipoda Phronima sp. 2 (6) 17–23 TL lomeso.dvm 5, 6 400–975 0.00 Ϯ 0.00 Order Decapoda Crab Megalopae (unidentified) 3 (14) 3–14 CL epi 7, 8 0–200 0.94 Ϯ 1.63 Janicella spinacauda 7 (10) 7–16 CL upmeso.dvm 9 500–600 30.39 Ϯ 23.82 Lobster Phyllosoma (unidentified) 5 42–67 CL upmeso.dvm 10 80–400 18.54 Ϯ 13.61 Oplophorus gracilirostris 5 9–20 CL upmeso.dvm 9, 11 500–650 90.23 Ϯ 103.20 Sergestes sp. 5 8–25 CL upmeso.dvm 12, 13 200–600 45.61 Ϯ 51.29 Sergia sp. 5 6–10 CL upmeso.dvm 12, 13 300–600 0.45 Ϯ 1.01 Systellapsis sp. 5 5–44 CL lomeso.dvm 9, 12 600–1100 22.63 Ϯ 38.18 Order Euphausiacea Euphausiids (unidentified) 2 (7) 5–7 CL upmeso.dvm 14, 15 400–600 7.72 Ϯ 10.92 Order Isopoda Anuropus sp. -

Atlas Over Danske Saltvandsfisk Nordisk Beryx
Atlas over danske saltvandsfisk Nordisk beryx Beryx decadactylus Cuvier, 1829 Af Henrik Carl Nordisk beryx på 48,5 cm fanget på Bøchers Banke, 17. november 2010 © Henrik Carl. Projektet er finansieret af Aage V. Jensen Naturfond Alle rettigheder forbeholdes. Det er tilladt at gengive korte stykker af teksten med tydelig kildehenvisning. Teksten bedes citeret således: Carl, H. 2019. Atlas over danske saltvandsfisk – Nordisk beryx. Statens Naturhistoriske Museum. Online-udgivelse, februar 2019. Systematik og navngivning Der er gennem tiden beskrevet ti arter i slægten Beryx Cuvier, 1829. Tre af disse er siden flyttet til familiens anden slægt Centroberyx, og fire arter har vist sig at være synonymer af nordisk beryx. Slægten består nu således af tre arter, hvoraf de to findes i europæiske farvande. Foruden nordisk beryx drejer det sig om pragt-beryx, Beryx splendens. Sidstnævnte kendes ikke fra dansk farvand, men blev fanget ved Sverige i 2011 (Jonsson 2012). Ynglen har tidligere været beskrevet under slægtsnavnet Actinoberyx Roule, 1923. Det officielle navn er nordisk beryx – et navn, der er blevet brugt siden midt i 1900-tallet (Bruun & Pfaff 1950). Før den tid havde fisken ikke noget dansk navn. Slægtsnavnet Beryx stammer fra det antikke Grækenland, hvor det var navnet på en ukendt fiskeart. Artsnavnet decadactylus betyder tifingret, hvilket hentyder til, at arten normalt har ti bugfinnestråler (Kullander & Delling 2012). Udseende og kendetegn Kroppen er høj og sammentrykt. Den største højde udgør 44-50 % af standardlængden (længden fra snuden til basis af halefinnen) (Maul 1986). Hovedet er stort, og munden er ligeledes stor med et tydeligt underbid. Munden kan spiles vidt op.