Radiation and Transoceanic Dispersal of the Schoenoid Sedges
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
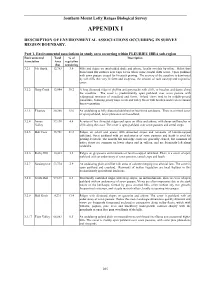
A Biological Survey of the Southern Mount Lofty Ranges
Southern Mount Lofty Ranges Biological Survey APPENDIX I DESCRIPTION OF ENVIRONMENTAL ASSOCIATIONS OCCURRING IN SURVEY REGION BOUNDARY. Part 1. Environmental associations in study area occurring within FLEURIEU IBRA sub-region Environmental Total % of Description Association Area vegetation (ha) remaining 3.2.1 Mt. Rapid 12,763 3.9 Hills and ridges on interbedded shale and arkose, locally overlain by tillite. Relict fans form broad flat surfaces near Cape Jervis where some coastal cliffs occur. Open parkland with sown pasture is used for livestock grazing. The scenery of the coastline is dominated by tall cliffs that vary in form and steepness, the amount of rock outcrop and vegetative cover. 3.2.2 Deep Creek 12,984 30.2 A long dissected ridge of phyllite and greywacke with cliffs, or beaches and dunes along the coastline. The cover is predominantly open parkland over sown pasture with widespread remnants of woodland and forest. Inland views tend to be middle-ground panoramic, featuring grassy ridge crests and valley floors with bracken and reed or remnant forest vegetation. 3.2.3 Fleurieu 30,389 15.6 An undulating to hilly dissected tableland on lateritized sandstone. There is a mixed cover of open parkland, forest plantation and woodland. 3.2.4 Inman 37,130 4.4 A series of low dissected ridges and spurs on tillite and arkose, with dunes and beaches or Valley cliffs along the coast. The cover is open parkland over sown pastures and cereal crops. 3.2.5 Bob Tiers 15,761 21.3 Ridges on schist and gneiss with dissected slopes and remnantsof laterite-capped tableland. -

Island Biology Island Biology
IIssllaanndd bbiioollooggyy Allan Sørensen Allan Timmermann, Ana Maria Martín González Camilla Hansen Camille Kruch Dorte Jensen Eva Grøndahl, Franziska Petra Popko, Grete Fogtmann Jensen, Gudny Asgeirsdottir, Hubertus Heinicke, Jan Nikkelborg, Janne Thirstrup, Karin T. Clausen, Karina Mikkelsen, Katrine Meisner, Kent Olsen, Kristina Boros, Linn Kathrin Øverland, Lucía de la Guardia, Marie S. Hoelgaard, Melissa Wetter Mikkel Sørensen, Morten Ravn Knudsen, Pedro Finamore, Petr Klimes, Rasmus Højer Jensen, Tenna Boye Tine Biedenweg AARHUS UNIVERSITY 2005/ESSAYS IN EVOLUTIONARY ECOLOGY Teachers: Bodil K. Ehlers, Tanja Ingversen, Dave Parker, MIchael Warrer Larsen, Yoko L. Dupont & Jens M. Olesen 1 C o n t e n t s Atlantic Ocean Islands Faroe Islands Kent Olsen 4 Shetland Islands Janne Thirstrup 10 Svalbard Linn Kathrin Øverland 14 Greenland Eva Grøndahl 18 Azores Tenna Boye 22 St. Helena Pedro Finamore 25 Falkland Islands Kristina Boros 29 Cape Verde Islands Allan Sørensen 32 Tristan da Cunha Rasmus Højer Jensen 36 Mediterranean Islands Corsica Camille Kruch 39 Cyprus Tine Biedenweg 42 Indian Ocean Islands Socotra Mikkel Sørensen 47 Zanzibar Karina Mikkelsen 50 Maldives Allan Timmermann 54 Krakatau Camilla Hansen 57 Bali and Lombok Grete Fogtmann Jensen 61 Pacific Islands New Guinea Lucía de la Guardia 66 2 Solomon Islands Karin T. Clausen 70 New Caledonia Franziska Petra Popko 74 Samoa Morten Ravn Knudsen 77 Tasmania Jan Nikkelborg 81 Fiji Melissa Wetter 84 New Zealand Marie S. Hoelgaard 87 Pitcairn Katrine Meisner 91 Juan Fernandéz Islands Gudny Asgeirsdottir 95 Hawaiian Islands Petr Klimes 97 Galápagos Islands Dorthe Jensen 102 Caribbean Islands Cuba Hubertus Heinicke 107 Dominica Ana Maria Martin Gonzalez 110 Essay localities 3 The Faroe Islands Kent Olsen Introduction The Faroe Islands is a treeless archipelago situated in the heart of the warm North Atlantic Current on the Wyville Thompson Ridge between 61°20’ and 62°24’ N and between 6°15’ and 7°41’ W. -

Ficha Catalográfica Online
UNIVERSIDADE ESTADUAL DE CAMPINAS INSTITUTO DE BIOLOGIA – IB SUZANA MARIA DOS SANTOS COSTA SYSTEMATIC STUDIES IN CRYPTANGIEAE (CYPERACEAE) ESTUDOS FILOGENÉTICOS E SISTEMÁTICOS EM CRYPTANGIEAE CAMPINAS, SÃO PAULO 2018 SUZANA MARIA DOS SANTOS COSTA SYSTEMATIC STUDIES IN CRYPTANGIEAE (CYPERACEAE) ESTUDOS FILOGENÉTICOS E SISTEMÁTICOS EM CRYPTANGIEAE Thesis presented to the Institute of Biology of the University of Campinas in partial fulfillment of the requirements for the degree of PhD in Plant Biology Tese apresentada ao Instituto de Biologia da Universidade Estadual de Campinas como parte dos requisitos exigidos para a obtenção do Título de Doutora em Biologia Vegetal ESTE ARQUIVO DIGITAL CORRESPONDE À VERSÃO FINAL DA TESE DEFENDIDA PELA ALUNA Suzana Maria dos Santos Costa E ORIENTADA PELA Profa. Maria do Carmo Estanislau do Amaral (UNICAMP) E CO- ORIENTADA pelo Prof. William Wayt Thomas (NYBG). Orientadora: Maria do Carmo Estanislau do Amaral Co-Orientador: William Wayt Thomas CAMPINAS, SÃO PAULO 2018 Agência(s) de fomento e nº(s) de processo(s): CNPq, 142322/2015-6; CAPES Ficha catalográfica Universidade Estadual de Campinas Biblioteca do Instituto de Biologia Mara Janaina de Oliveira - CRB 8/6972 Costa, Suzana Maria dos Santos, 1987- C823s CosSystematic studies in Cryptangieae (Cyperaceae) / Suzana Maria dos Santos Costa. – Campinas, SP : [s.n.], 2018. CosOrientador: Maria do Carmo Estanislau do Amaral. CosCoorientador: William Wayt Thomas. CosTese (doutorado) – Universidade Estadual de Campinas, Instituto de Biologia. Cos1. Savanas. 2. Campinarana. 3. Campos rupestres. 4. Filogenia - Aspectos moleculares. 5. Cyperaceae. I. Amaral, Maria do Carmo Estanislau do, 1958-. II. Thomas, William Wayt, 1951-. III. Universidade Estadual de Campinas. Instituto de Biologia. IV. Título. -

Sand Mine Near Robertson, Western Cape Province
SAND MINE NEAR ROBERTSON, WESTERN CAPE PROVINCE BOTANICAL STUDY AND ASSESSMENT Version: 1.0 Date: 06 April 2020 Authors: Gerhard Botha & Dr. Jan -Hendrik Keet PROPOSED EXPANSION OF THE SAND MINE AREA ON PORTION4 OF THE FARM ZANDBERG FONTEIN 97, SOUTH OF ROBERTSON, WESTERN CAPE PROVINCE Report Title: Botanical Study and Assessment Authors: Mr. Gerhard Botha and Dr. Jan-Hendrik Keet Project Name: Proposed expansion of the sand mine area on Portion 4 of the far Zandberg Fontein 97 south of Robertson, Western Cape Province Status of report: Version 1.0 Date: 6th April 2020 Prepared for: Greenmined Environmental Postnet Suite 62, Private Bag X15 Somerset West 7129 Cell: 082 734 5113 Email: [email protected] Prepared by Nkurenkuru Ecology and Biodiversity 3 Jock Meiring Street Park West Bloemfontein 9301 Cell: 083 412 1705 Email: gabotha11@gmail com Suggested report citation Nkurenkuru Ecology and Biodiversity, 2020. Section 102 Application (Expansion of mining footprint) and Final Basic Assessment & Environmental Management Plan for the proposed expansion of the sand mine on Portion 4 of the Farm Zandberg Fontein 97, Western Cape Province. Botanical Study and Assessment Report. Unpublished report prepared by Nkurenkuru Ecology and Biodiversity for GreenMined Environmental. Version 1.0, 6 April 2020. Proposed expansion of the zandberg sand mine April 2020 botanical STUDY AND ASSESSMENT I. DECLARATION OF CONSULTANTS INDEPENDENCE » act/ed as the independent specialist in this application; » regard the information contained in this -

Can Isoprenoids in Leaves and Roots of Plants Serve As Biomarkers for Past Vegetation Changes? a Case Study from the Ecuadorian Andes
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Springer - Publisher Connector Plant Soil (2007) 291:181–198 DOI 10.1007/s11104-006-9185-1 ORIGINAL PAPER Can isoprenoids in leaves and roots of plants serve as biomarkers for past vegetation changes? A case study from the Ecuadorian Andes Boris Jansen Æ Klaas G. J. Nierop Æ Femke H. Tonneijck Æ Frans W. M. van der Wielen Æ Jacobus M. Verstraten Received: 18 October 2006 / Accepted: 15 December 2006 / Published online: 17 January 2007 Ó Springer Science+Business Media B.V. 2007 Abstract Large-scale clear-cutting and burning suitable records. Such biomarkers could help caused the altitude of the natural upper forest line establish past vegetation dynamics including the (UFL) in the Northern Ecuadorian Andes to UFL position. For an isoprenoid to be a biomar- decline to the point that its ‘natural’ position is ker in soils normally it must be absent from the now uncertain. To obtain a detailed reconstruc- roots of a plant species as roots do not enter soils tion of the dynamics of the UFL over the last few in chronological order. For peat deposits this thousand years, traditional proxies alone do not criteria only needs to be met for the peat species suffice. For instance, pollen analysis suffers from themselves as only roots from peat species will be a low altitudinal resolution due to the large wind- present. Two diterpenes, four phytosterols and six blown component. In an attempt to find new, pentacyclic triterpenoids met the criteria for additional proxies to study past UFL dynamics in biomarker in peat records. -

Diversidad De Plantas Y Vegetación Del Páramo Andino
Plant diversity and vegetation of the Andean Páramo Diversidad de plantas y vegetación del Páramo Andino By Gwendolyn Peyre A thesis submitted for the degree of Doctor from the University of Barcelona and Aarhus University University of Barcelona, Faculty of Biology, PhD Program Biodiversity Aarhus University, Institute of Bioscience, PhD Program Bioscience Supervisors: Dr. Xavier Font, Dr. Henrik Balslev Tutor: Dr. Xavier Font March, 2015 Aux peuples andins Summary The páramo is a high mountain ecosystem that includes all natural habitats located between the montane treeline and the permanent snowline in the humid northern Andes. Given its recent origin and continental insularity among tropical lowlands, the páramo evolved as a biodiversity hotspot, with a vascular flora of more than 3400 species and high endemism. Moreover, the páramo provides many ecosystem services for human populations, essentially water supply and carbon storage. Anthropogenic activities, mostly agriculture and burning- grazing practices, as well as climate change are major threats for the páramo’s integrity. Consequently, further scientific research and conservation strategies must be oriented towards this unique region. Botanical and ecological knowledge on the páramo is extensive but geographically heterogeneous. Moreover, most research studies and management strategies are carried out at local to national scale and given the vast extension of the páramo, regional studies are also needed. The principal limitation for regional páramo studies is the lack of a substantial source of good quality botanical data covering the entire region and freely accessible. To meet the needs for a regional data source, we created VegPáramo, a floristic and vegetation database containing 3000 vegetation plots sampled with the phytosociological method throughout the páramo region and proceeding from the existing literature and our fieldwork (Chapter 1). -

Vegetation of Andean Wetlands (Bofedales) in Huascarán National Park, Peru
Vegetation of Andean wetlands (bofedales) in Huascarán National Park, Peru M.H. Polk1, K.R. Young1, A. Cano2 and B. León1,2 1Department of Geography & the Environment, The University of Texas at Austin, USA 2Museo de Historia Natural, Universidad Nacional Mayor de San Marcos, Lima, Perú _______________________________________________________________________________________ SUMMARY Hybrid terrestrial-aquatic ecosystems in the Andes, commonly known as bofedales, consist of both peatlands and wet meadows and line valley floors at elevations > 3800 m. Compared with similar ecosystems at lower altitudes and higher latitudes, the ecosystem processes and spatial patterns of bofedales are only just beginning to be understood. The research presented here provides the first exploratory and descriptive analysis of the biodiversity and place-to-place variation of vegetation in bofedales in three valleys inside Peru’s Huascarán National Park. Through vegetation surveys, we recorded 112 plant species in 29 families. Over a short geographical distance, a valley-to-valley comparison showed high dissimilarity in terms of species composition. Based on dominant life form and species composition, vegetation in bofedales can be grouped into five assemblages. Our preliminary analysis suggests that several abiotic factors could influence the floristic composition of bofedales: elevation, bulk density, percent organic matter, and cation exchange capacity. The findings of high valley-to-valley variation in species, soil and elevation influences may be useful to land managers of high mountain landscapes that are undergoing transformation related to glacier recession. While our findings advance research on tropical Andean bofedales, they also highlight the need for additional comprehensive investigations to fill gaps in knowledge about the tropical mountains of Latin America. -

Genetic Diversity in the Andes
Edinburgh Research Explorer Genetic diversity in the Andes Citation for published version: Gómez-Gutiérrez, MC, Pennington, RT, Neaves, LE, Milne, RI, Madriñán, S & Richardson, JE 2017, 'Genetic diversity in the Andes: variation within and between the South American species of Oreobolus R. Br. (Cyperaceae)', Alpine Botany, vol. 127, no. 2, pp. 155-170. https://doi.org/10.1007/s00035-017-0192-z Digital Object Identifier (DOI): 10.1007/s00035-017-0192-z Link: Link to publication record in Edinburgh Research Explorer Document Version: Peer reviewed version Published In: Alpine Botany Publisher Rights Statement: © Swiss Botanical Society 2017 The final publication is available at link.springer.com via http://dx.doi.org/10.1007/s00035-017-0192-z General rights Copyright for the publications made accessible via the Edinburgh Research Explorer is retained by the author(s) and / or other copyright owners and it is a condition of accessing these publications that users recognise and abide by the legal requirements associated with these rights. Take down policy The University of Edinburgh has made every reasonable effort to ensure that Edinburgh Research Explorer content complies with UK legislation. If you believe that the public display of this file breaches copyright please contact [email protected] providing details, and we will remove access to the work immediately and investigate your claim. Download date: 23. Sep. 2021 Manuscript Click here to download Manuscript Gomez-Gutierrez et al Genetic diversity in Oreobolus R2.docx Click here to view linked References 1 2 3 4 1 GENETIC DIVERSITY IN THE ANDES: 5 6 2 VARIATION WITHIN AND BETWEEN THE 7 8 3 SOUTH AMERICAN SPECIES OF OREOBOLUS 9 10 4 R. -

Using the Checklist N W C
Using the checklist • The arrangement of the checklist is alphabetical by family followed by genus, grouped under Pteridophyta, Gymnosperms, Monocotyledons and Dicotyledons. • All species and synonyms are arranged alphabetically under genus. • Accepted names are in bold print while synonyms or previously-used names are in italics. • In the case of synonyms, the currently used name follows the equals sign (=), and only refers to usage in Zimbabwe. • Distribution information is included under the current name. • The letters N, W, C, E, and S, following each listed taxon, indicate the known distribution of species within Zimbabwe as reflected by specimens in SRGH or cited in the literature. Where the distribution is unknown, we have inserted Distr.? after the taxon name. • All species known or suspected to be fully naturalised in Zimbabwe are included in the list. They are preceded by an asterisk (*). Species only known from planted or garden specimens were not included. Mozambique Zambia Kariba Mt. Darwin Lake Kariba N Victoria Falls Harare C Nyanga Mts. W Mutare Gweru E Bulawayo GREAT DYKEMasvingo Plumtree S Chimanimani Mts. Botswana N Beit Bridge South Africa The floristic regions of Zimbabwe: Central, East, North, South, West. A checklist of Zimbabwean vascular plants A checklist of Zimbabwean vascular plants edited by Anthony Mapaura & Jonathan Timberlake Southern African Botanical Diversity Network Report No. 33 • 2004 • Recommended citation format MAPAURA, A. & TIMBERLAKE, J. (eds). 2004. A checklist of Zimbabwean vascular plants. -

Falkland Islands Species List
Falkland Islands Species List Day Common Name Scientific Name x 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 1 BIRDS* 2 DUCKS, GEESE, & WATERFOWL Anseriformes - Anatidae 3 Black-necked Swan Cygnus melancoryphus 4 Coscoroba Swan Coscoroba coscoroba 5 Upland Goose Chloephaga picta 6 Kelp Goose Chloephaga hybrida 7 Ruddy-headed Goose Chloephaga rubidiceps 8 Flying Steamer-Duck Tachyeres patachonicus 9 Falkland Steamer-Duck Tachyeres brachypterus 10 Crested Duck Lophonetta specularioides 11 Chiloe Wigeon Anas sibilatrix 12 Mallard Anas platyrhynchos 13 Cinnamon Teal Anas cyanoptera 14 Yellow-billed Pintail Anas georgica 15 Silver Teal Anas versicolor 16 Yellow-billed Teal Anas flavirostris 17 GREBES Podicipediformes - Podicipedidae 18 White-tufted Grebe Rollandia rolland 19 Silvery Grebe Podiceps occipitalis 20 PENGUINS Sphenisciformes - Spheniscidae 21 King Penguin Aptenodytes patagonicus 22 Gentoo Penguin Pygoscelis papua Cheesemans' Ecology Safaris Species List Updated: April 2017 Page 1 of 11 Day Common Name Scientific Name x 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 23 Magellanic Penguin Spheniscus magellanicus 24 Macaroni Penguin Eudyptes chrysolophus 25 Southern Rockhopper Penguin Eudyptes chrysocome chrysocome 26 ALBATROSSES Procellariiformes - Diomedeidae 27 Gray-headed Albatross Thalassarche chrysostoma 28 Black-browed Albatross Thalassarche melanophris 29 Royal Albatross (Southern) Diomedea epomophora epomophora 30 Royal Albatross (Northern) Diomedea epomophora sanfordi 31 Wandering Albatross (Snowy) Diomedea exulans exulans 32 Wandering -

Parque Nacional Tierra Del Fuego Flora
Parque Nacional Tierra del Fuego Flora • Common English Name (Nombre Español o Local)Order Family Genus species) Monocotyledons (Monocotyledones) • Arrowgrass, Marsh (??) (Najadales Juncaginaceae Triglochin palustris) • Arrowgrass, Seaside (??) (Najadales Juncaginaceae Triglochin maritima) • Bentgrass, Common (Pasto Quila) (Poales Gramineae/Poaceae Agrostis capillaris) • Bentgrass, Upland (??) (Poales Gramineae/Poaceae Agrostis perennans) • Bluegrass (??) (Poales Gramineae/Poaceae Poa alopecurus) • Bluegrass (??) (Poales Gramineae/Poaceae Poa breviculmis) • Bluegrass (??) (Poales Gramineae/Poaceae Poa rigidifolia) • Bluegrass (??) (Poales Gramineae/Poaceae Poa scaberula) • Bluegrass (Möra-Shúka) (Poales Gramineae/Poaceae Poa yaganica) • Bluegrass, Annual (Pastito de Invierno) (Poales Gramineae/Poaceae Poa annua) • Bluegrass, Canada (??) (Poales Gramineae/Poaceae Poa compressa) • Bluegrass, Kentucky (Pasto de Mallin) (Poales Gramineae/Poaceae Poa pratensis) • Bluegrass, Northern (??) (Poales Gramineae/Poaceae Poa stenantha) • Bulrush, California (Junco) (Cyperales Cyperaceae Schoenoplectus californicus) • Bulrush, Nevada (Scirpus) (Cyperales Cyperaceae Amphiscirpus nevadensis) • Foxtail, Meadow (Alopecuro de los Prados-cola de Zorro) (Poales Gramineae/Poaceae Alopecurus pratensis) • Grass, Black (??) (Poales Gramineae/Poaceae Alopecurus magellanicus) • Grass, Fiber Optic (??) (Cyperales Cyperaceae Isolepis cernua) • Grass, Small Tussock (??) (Poales Gramineae/Poaceae Festuca magellanica) • Grass, Sweet Holy (Ratonera) (Poales Gramineae/Poaceae -

Phylogeny Reconstruction of the Schoeneae (Cyperaceae) with a Focus on Southern-African Genera
BOLUS LIBRAR Y C31 0000 0687 Phylogeny reconstruction of the Schoeneae (Cyperaceae) with a focus on southern-African genera By: Jessica Henning Supervisors: Dr A.M. Muasya Dr G.A. Verboom University of Cape Town A dissertation submitted to the University of Cape Town, in partial fulfilment of the requirements for the award of an Honours degree in Botany. OCTOBER 2008 The copyright of this thesis vests in the author. No quotation from it or information derived from it is to be published without full acknowledgement of the source. The thesis is to be used for private study or non- commercial research purposes only. Published by the University of Cape Town (UCT) in terms of the non-exclusive license granted to UCT by the author. University of Cape Town Plagiarism declaration I know that plagiarism is a serious form of academic dishonesty. I have read the document about avoiding plagiarism, I am familiar with its contents and I have avoided all forms of plagiarism mentioned there. Where I have used the words of others, I have indicated this by the use of quotation marks. I have referenced all quotations and other ideas borrowed from others. I have not and shall not allow others to plagiarise my work. Signed: Date: 2.9J \0 { �� Abstract In this study both plastid and nuclear DNA sequences (rbcL, trnL-trnF, rps16, ITS and ETS) were analysed. New sequences were added to the matrix from Verboom (2006). Parsimony method was used for phylogeny reconstruction. Morphological characters were then optimised on the parsimony tree using both maximum likelihood and parsimony reconstruction.