A Study on Earthworm Population and Diversity with Special Reference to Physicochemical Parameters in Different Habitats of South 24 Parganas District in West Bengal
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

History of India
HISTORY OF INDIA VOLUME - 2 History of India Edited by A. V. Williams Jackson, Ph.D., LL.D., Professor of Indo-Iranian Languages in Columbia University Volume 2 – From the Sixth Century B.C. to the Mohammedan Conquest, Including the Invasion of Alexander the Great By: Vincent A. Smith, M.A., M.R.A.S., F.R.N.S. Late of the Indian Civil Service, Author of “Asoka, the Buddhist Emperor of India” 1906 Reproduced by Sani H. Panhwar (2018) Preface by the Editor This volume covers the interesting period from the century in which Buddha appeared down to the first centuries after the Mohammedans entered India, or, roughly speaking, from 600 B.C. to 1200 A.D. During this long era India, now Aryanized, was brought into closer contact with the outer world. The invasion of Alexander the Great gave her at least a touch of the West; the spread of Buddhism and the growth of trade created new relations with China and Central Asia; and, toward the close of the period, the great movements which had their origin in Arabia brought her under the influences which affected the East historically after the rise of Islam. In no previous work will the reader find so thorough and so comprehensive a description as Mr. Vincent Smith has given of Alexander’s inroad into India and of his exploits which stirred, even if they did not deeply move, the soul of India; nor has there existed hitherto so full an account of the great rulers, Chandragupta, Asoka, and Harsha, each of whom made famous the age in which he lived. -
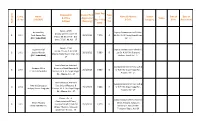
Comp. Sl. No Name S/D/W/O Designation & Office Address Date of First Application (Receving) Basic Pay / Pay in Pay Band Type
Basic Pay Designation Date of First Type Comp. Name / Name & Address Roster Date of Date of & Office Application of Status Sl. No S/D/W/O Pay in Pay D.D.O. Category Birth Retirement Address (Receving) Flat Waiting NoWaiting Band Sepoy - 2969 Anirban Roy Deputy Commissioner of Police, Deputy Commissioner of 1 2091 Asok Kumar Roy 29/1/2013 7,370 D 6th Bn. K.A.P. Body Guard Lines, Police, 6th Bn. K.A.P. B.G. (D.C. Submitted) Kol - 27 Lines, 7, D.H. Rd. Kol - 27 Sepoy - 2166 Sujan Mandal Deputy commissioner of Police, 1st Bn. 'E' Coy. 7, D.H. Rd. 2 2092 Jaydev Mandal 29/1/2013 7,980 D 1st Bn. K.A.P 7th Diamond Alipore, Body Guard Lines, Kol - (D.C. Submitted) Harbour Road, Kol - 27 27 Lower Division Assistant Assistant Director ( H.Q.) DLR & Suvendu Mitra Director of Land Records & 3 2093 29/1/2013 7,080 D S, W.B. 35, Gopal Nagar Rd., Lt. Anil Baran Mitra Surveys, W.B. 35, Gopal Nagar Alipore, Kol - 27 Rd., Alipore, Kol - 27 Lower Division Assistant Assistant Director ( H.Q.) DLR & Amardip Sengupta Dte. Of Land Records & 4 2094 29/1/2013 7,080 D S, W.B. 35, Gopal Nagar Rd., Sri Ajoy Kumar Sengupta Surveys, W. B. 35, Gopal Nagar Alipore, Kol - 27 Rd., Alipore, Kol - 27 Driver - Gr. - II Administrative Officer, Pool Car Administrative Officer ( Shyam Murmu Office,7 &11/1, Ashutosh 5 2097 Transport Deptt.) Pool Car 29/1/2013 7,370 D Chutu Ram Murmu Mukherjee Road, Home Office, 7 & 11/1, Ashutosh Transport Deptt., Kol-20 Mukherjee Rd. -

1 Volume-Xiii West Bengal
CENSUS OF INDIA 1991 REGIONAL DIVISIONS OF INDIA -A CARTOGRAPHIC ANALYSIS OCCASIONAL PAPERS SERIES -1 VOLUME-XIII WEST BENGAL Planning & Supervisipn Dr. R. P. Singh . Assistant Registrar General (Map) General Direction & Editing J. K. BANTHIA of the Indian Administrative Service Registrar General & Census Commissioner, India . 2A, Mansingh Road, New Delhi * Maps included in this volume are based upon Survey of India map with the pellnission of the Surveyor General ofIndia. * The Territorial water ofIndia extend into the sea to a distance of twelve nautical miles measured from the appropriate base line. * The inter-state boundaries between Arunachal Pradesh, Assam and Meghataya shown on this map are as interpreted from the North-Eastem Areas (Reorganisation) Act, 1971 but have yet to be verified. (For India Map only) * Government oflndia Copyright, 2001. FOREWORD "Regional Divisions of India ~ A Cartographic Analysis", of each State and Union Temtory Volume,1991 is undertaken by the Map Division of this office. The project is in continuation ofa similar 'Occasional'Paper' published during the 1981 Census. In this study, regions are delineated at macro, messo, micro and sub-micro level in e~h state/.union terril:ory. While delineating such regions, the physio-geographic factors are taken into accclunt. These physio-geographic factors consist of (i) Physiography; (ii) Geological strui;ture; (iii) Forest coverage; (iv) Climatic conditions and (v) Soils. The sub"micro regions Yare delineated within the district keeping in view the above physio-geographic factors. The regi.onal boundaries delineated earlier at different levels are retained as such. However, the jurisdictional changes at the district level during 1981-91 were added in the respective regions. -
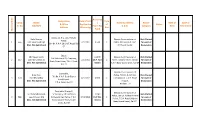
W Aitin G Sl No Comp. Sl. No Name S/D/W/O Designation & Office Address Date of First Application
Basic Pay Designation Date of First Type Comp. Name / Name & Address Roster Date of Date of & Office Application of Status Sl. No S/D/W/O Pay in Pay D.D.O. Category Birth Retirement Address (Receving) Flat Band Waiting Sl No Sepoy-32, H.Q. Coy. Kolkata Kaila Sherpa, Deputy Commissioner of Not Allotted Police, 1 666 Late Kancha Sherpa 2/1/2012 8,570 C Police, 8th Bn.K.A.P. 29/1 for want of 8th Bn. K.A.P. 29/1 B.T.Road, Kol- (Dec. Not Submitted) B.T.Road, Kol-02 Dealaration 02 Sepoy, Mir Rejaul Karim, 12,240 Deputy Commissioner of Not Allotted 3rd Battalian H.Q. Company, 2 667 Late Mir Ershad Ali 2/1/2012 (G.P. Not C Police, Kolkata Police, 3rd Bn. for want of Body Guard Lines, 7 D.H. Road, (Dec. Not Submitted) Given) K.A.P. Body Guard Lines, Kol-27 Dealaration Kol-27 Deputy Commissioner of Constable, Utpal Roy, Police, 7th Bn. K.A.P. B.G. Not Allotted 7th Bn. K.A.P. Body Guard 3 670 Banchhalal Roy 4/1/2012 8,920 C Lines,(Alipur) 7, D.H. Road for want of Lines(Alipur), (Dec. Not Submitted) Alipore, Dealaration 7 D.H. Road, Kol-27 Kolkata - 27 Constable (Group-C) Deputy Commissioner of Sri Chakradhari Mal, 'E' Company 7th Battalion 12240 Not Allotted Police, 7th Bn. Kolkata Armed 4 689 Late Nilratan Mal Kolkata Armed Police, 7 D.H. 24/1/2012 (G.P.Not C for want of Police, 7 D.H. -

Wbcs Prelims 2020
GEOGRAPHY for WBCS PRELIMS 2020 JITIN YADAV, IAS 1 GEOGRAPHY NOTES FOR WBCS PRELIMS 2020 BY JITIN YADAV, IAS CONTENTS S. No. TOPIC PAGE 1. West Bengal Geography 3 2. WB Location and ExtEnsion 3 3. Census of WB 4 4. Physiography of WB 5 5. RivEr of WB 8 6. Soil of WB 11 7. MinErals in WB 11 8. Industry in WB 12 9. Natural VEgEtation & AgriculturE in WB 13 10. HydropoWEr projEcts & Dams in WB 14 11. Economy in WB 15 12. National Parks, Bird and Wild LifE 15 Sanctuaries and Biospheres in WB etc 13. WB SchEmEs 16 14. Indian Geography 18 15. Geological structurEs of India 19 16. Physiography of India 20 17. Soils of India 27 18. VegEtation 30 19. AgriculturE and Crops 32 20. DrainagE SystEm 40 21. Lakes 47 22. MultipurposE ProjEct 50 23. ClimatE 55 2 GEOGRAPHY NOTES FOR WBCS PRELIMS 2020 BY JITIN YADAV, IAS WEST BENGAL GEOGRAPHY LOCATION AND EXTENSION • Located in East India • BORDERS Direction Country/State Boundary/Border(km) East BangladEsh 2217 North Bhutan 150 North Sikkim 60 North West Nepal 90 North East Assam 90 West Jharkhand 800 West Bihar 300 South West Odisha 150 South Bay of Bengal - o Total 9 districts shares their boundary with Bangladesh o Odisha is thE largEst nEighbouring statE of WB in tErms of arEa • EXTENSION o North – South = 623km o East – WEst =320km o Chicken’s Neck - NarroWEst part, is 9km in lEngth, locatEd at Chopra in Uttar Dinajpur o Longitude = 85050’ E and 890 50’ E o Latitude = 27013’ N and 210 25’ N o Tropic of Cancer passes through § Nadia § Purba Bardhman § Paschim Bardhman § Bankura § Purulia o Total area -
West Bengal Police Directorate Writers’ Buildings 2 Nd, Floor, Ii Nd Block Kolkata - 700 001
MANUAL – 9 DIRECTORY OF OFFICER & EMPLOYEES SECTION- 4(1)(B)(IX) WEST BENGAL POLICE DIRECTORATE WRITERS’ BUILDINGS 2 ND, FLOOR, II ND BLOCK KOLKATA - 700 001 CONTROL ROOM – 2214 4031 UNITS / OFFICER LOCATION OF OFFICE STD OFFICE NOS. DG & IGP WB WRITERS’ BUILDINGS, KOLKATA - 700 001 033 2214-5400 2214-5486 ( FAX ) ADG ( ADMN.) WB WRITERS’ BUILDINGS, KOLKATA - 700 001 033 2214-4038 ADG ( MOD & CORD ) WB WRITERS’ BUILDINGS, KOLKATA - 700 001 033 2214-4416 IGP ( HQRS. ) WB WRITERS’ BUILDINGS, KOLKATA - 700 001 033 2214-3406 IGP ( ORG. ) WB WRITERS’ BUILDINGS, KOLKATA - 700 001 033 2214-4044 IGP ( ADMN. ) WB WRITERS’ BUILDINGS, KOLKATA - 700 001 033 2214-4041 IGP ( LAW & ORDER ) WB WRITERS’ BUILDINGS, KOLKATA - 700 001 033 2214-5401 / 5417 SPL. IGP & DIG ( HQRS.) WB WRITERS’ BUILDINGS, KOLKATA - 700 001 033 2214-5088 SPL. IGP & DIG (MOD & WRITERS’ BUILDINGS, KOLKATA - 700 001 033 2214-1261 CORD) WB SPL. IGP & DIG ( ADMN.) WB WRITERS’ BUILDINGS, KOLKATA - 700 001 033 2214-5009 SPL. IGP & DIG ( ORG. ) WB WRITERS’ BUILDINGS, KOLKATA - 700 001 033 2214-5537 DIG ( IPS CELL ) WB WRITERS’ BUILDINGS, KOLKATA - 700 001 033 2214-4778 DIG ( P & W ) WB WRITERS’ BUILDINGS, KOLKATA - 700 001 033 2214-1198 AIG ( S ) WB WRITERS’ BUILDINGS, KOLKATA - 700 001 033 2214-4042 DY. SP, DGP’S CONTROL WRITERS’ BUILDINGS, KOLKATA - 700 001 033 2214-5823 ROOM DGP’S CONTROL ROOM WRITERS’ BUILDINGS, KOLKATA - 700 001 033 2214-4031 2214-5486 ( FAX ) POLICE DIRECTORATE PBX WRITERS’ BUILDINGS, KOLKATA - 700 001 033 2214-5411 TO 5416 INTELIGENCE BRANCH 13, LORD SINHA ROAD, KOLKATA - 700 071. -
Council of Homoeopathic Medicine, Wb
COUNCIL OF HOMOEOPATHIC MEDICINE, W.B. Part B of the Register of Registered Practitioners Sl Reg. Date of Reg. Name Address Oualification No. No.. 1 B1 02-May-68 Dr. Nalini Kanta Ghosh 765B, Block 'P', New Alipore, Kol - 53 Prac.Quali 2 B5 02-May-68 Dr. Desha Priya Chatterjee 55/16, Purba Sinthee Road, Kol - 30 Prac.Quali 3 B6 02-May-68 Dr. Gobindo Prasad Mondal 17, Beniatala Lane, P.O. - Amhrest Street, Kol - 9 Prac.Quali 4 B9 02-May-68 Dr. Santosh Kr. Paul P-364/2, Vidyasagar Sarani, P.O. - Barisha (700008) Prac.Quali 5 B10 02-May-68 Dr. Sunil Kr. Nath 50, Porui Main Rd., Behala, Kol - 61 Prac.Quali 6 B11 02-May-68 Dr. Ramani Mohan Chowdhury 110B, Narikeldanga Nath Road, Kol - 11 Prac.Quali 7 B12 02-May-68 Dr. Kedar Chandra Sinha 59 - Harish Chatterjee Street, Kol - 25 Prac.Quali 8 B14 02-May-68 Dr. Dwijendranath Bhattacharje 17, Strand Rd., Dakshineswar, Ariadah, Kol - 57 Prac.Quali 9 B15 02-May-68 Dr. Amulya Charan Maiti 206/1E, Bidhan Sarani Suite No. 2, Kol - 6 Prac.Quali 10 B16 02-May-68 Dr. Biswanath Pal Flat No. G5&6, Block No. 7, Poddar Park, Kol - 45 Prac.Quali 11 B17 02-May-68 Dr. Tarapada Adhikary N41/1 Paharpur Rd., Kol - 24 Prac.Quali 12 B18 02-May-68 Dr. Ramendra Mohan Kundu 20A, Ultadanga Rd., Kol - 4 Prac.Quali 13 B19 02-May-68 Dr. Hrishikesh Gupta 32, Ram Kamal Sen Lane, Kol - 7 Prac.Quali 14 B22 02-May-68 Dr. -
Cctv Camera Damaged Cctv Cameras
Notice Inviting e-Tender P a g e ||| 1 NIT [ NOTICE INVITING e -TENDER ] No. : WBKP/CP/NIT -21/RESTORATION OF CCTV/TEN, Dated : 16.09 .2020 e-Tender FOR RESTORATION OF DAMAGED CCTV CAMERAS UNDER LCMS PROJECT OF KOLKATA POLICE KOLKATA POLICE DIRECTORATE Tender Section, 18, Lalbazar Street, Kolkata – 700 001. Ph. : (033) 2250 5275 e-Mail : [email protected] 2 ||| P a g e Notice Inviting e-Tender TABLE OF CONTENTS NOTICE INVITING e-TENDER .............................................................................................................................. 3 PART – A 4 I. DEFINITIONS ........................................................................................................................................... 4 II. PRE-BID QUALIFICATIONS........................................................................................................................ 4 1. Company Registration : .................................................................................................................................. 4 2. Trade Licence/Professional Tax/IT Return : .................................................................................................... 4 3. Undertaking Regarding Blacklisting : .............................................................................................................. 4 4. Undertaking Regarding Acceptance of the Terms & Conditions :................................................................... 4 5. Insolvency/Criminal Activities : ..................................................................................................................... -

Volume5 Issue9(3)
Volume 5, Issue 9(3), September 2016 International Journal of Multidisciplinary Educational Research Published by Sucharitha Publications 8-43-7/1, Chinna Waltair Visakhapatnam – 530 017 Andhra Pradesh – India Email: [email protected] Website: www.ijmer.in Editorial Board Editor-in-Chief Dr.K. Victor Babu Faculty, Department of Philosophy Andhra University – Visakhapatnam - 530 003 Andhra Pradesh – India EDITORIAL BOARD MEMBERS Prof. S.Mahendra Dev Prof. Fidel Gutierrez Vivanco Vice Chancellor Founder and President Indira Gandhi Institute of Development Escuela Virtual de Asesoría Filosófica Research Lima Peru Mumbai Prof. Igor Kondrashin Prof.Y.C. Simhadri The Member of The Russian Philosophical Vice Chancellor, Patna University Society Former Director The Russian Humanist Society and Expert of Institute of Constitutional and Parliamentary The UNESCO, Moscow, Russia Studies, New Delhi & Formerly Vice Chancellor of Dr. Zoran Vujisiæ Benaras Hindu University, Andhra University Rector Nagarjuna University, Patna University St. Gregory Nazianzen Orthodox Institute Universidad Rural de Guatemala, GT, U.S.A Prof. (Dr.) Sohan Raj Tater Former Vice Chancellor Singhania University, Rajasthan Prof.U.Shameem Department of Zoology Andhra University Visakhapatnam Prof.K.Sreerama Murty Department of Economics Dr. N.V.S.Suryanarayana Andhra University - Visakhapatnam Dept. of Education, A.U. Campus Vizianagaram Prof. K.R.Rajani Department of Philosophy Dr. Kameswara Sharma YVR Andhra University – Visakhapatnam Asst. Professor Dept. of Zoology Prof. P.D.Satya Paul Sri. Venkateswara College, Delhi University, Department of Anthropology Delhi Andhra University – Visakhapatnam I Ketut Donder Prof. Josef HÖCHTL Depasar State Institute of Hindu Dharma Department of Political Economy Indonesia University of Vienna, Vienna & Ex. Member of the Austrian Parliament Prof. -

Action Plan to Mitigate Flood, Cyclone & Water Logging 2021 the Kolkata
ACTION PLAN TO MITIGATE FLOOD, CYCLONE & WATER LOGGING 2021 THE KOLKATA MUNICIPAL CORPORATION 1 ESSENTIAL INFORMATION INCLUDING ACTION PLANS ARE MENTIONED UNDER FOLLOWING HEADS Sl No Item Page A Disaster Management- Introduction B Important Activities of K.M.C. in connection with the Disaster Management C Major Water Logging Pockets D Deployment of K.M.C. Mazdoor at Major Water Logging Pockets E ARRANGEMENT OF NIGHT GANG OF PARKS & SQUARES DEPARTMENT REQUIRED FOR REMOVAL OF UPROOTED TREES F LIST OF SEWERAGE AND DRAINAGE PUMPING STATIONS AND DEPLOYMENT OF TEMPORARY PORTABLE PUMPS DURING MONSOON G EMERGENCY ARRANGEMENT DURING THE ENSUING NOR’WESTER / RAINY SEASON IN THE NEXT FEW MONTHS OF 2021 (MPL.COMMR’S CIRCULAR NO. 01 OF 2021- 22 DT. 13/04/2021 H LIST OF THE ROADS WHERE CLEANING OF G.P.S MOUTHS / SWEEPING OF ROADS WILL BE MADE TWICE IN A DAY BY S.W.M. DEPARTMENT I ESSENTIAL TELEPHONE NUMBERS 2 A. DISASTER MANAGEMENT – INTRODUCTION The total area under Kolkata Municipal Corporation (KMC) is about 204.75 Sq. Km. which is divided into 16 Boroughs from Ward No-1 to Ward No-144. The total population of the KMC area as per 2011 Census is about 4.5 million. Moreover, the floating population of the city is about 6 million. They are coming to this city for their livelihood from the outskirt and suburbs of the city of Kolkata i.e. City of Joy. From the experience regarding the water logging/flood condition during rainy season for the last few years, the KMC authority felt to publicize the disaster management plan as well as disaster management system for the benefit of the citizens, local representatives, State Govt. -

Eastern Printing Group, Survey of India , 14, Wood
EASTERN PRINTING GROUP, SURVEY OF INDIA , 14, WOOD STREET, KOLKATA-16 AS ON 30-11-2017 Date of Date of joining Sl Present Father's/ Husband's/ Date of Appointment to Designation Group Prefix Employee Name Pay Level Pay (Rs.) of Present Contact No. Residential Adress Remarks No. Posting Mother's Name Birth the Govt. Grade Service Eastern Printing 21-Richie Road, Flat No. 8, 1 Director Group 'A' Shri Ashish Kaushal Ram Jiwan Kaushal (F) 13-08-73 03-08-00 L-13 138500 01-07-17 9830304561 Group , Kolkata Type-V, 4th Floor, Kolkata-19 Eastern Printing 29, Subhas Pally, 2 Junior Manager Group 'A' Shri Arin Kumar Dutta A.N. Dutta (F) 15-10-1962 28-01-1988 L-11 85800 04-12-15 9433622186 Group , Kolkata Kolkata-7000108 Eastern Printing Vill-Chikkampeth, P.O.- 3 Junior Manager Group 'A' Shri P. Kumar A. Perumal 13-05-76 23-12-98 L-10 77,700 20-07-16 9493975929 Group , Kolkata Muthampatti, Dist. Dharmapuri, Tamil Nadu-635301 B-12/402, A.E.C.H.S, Eastern Printing 4 Works Manager Group 'B' Shri Susanta Dhar Roy S.C. Dhar Roy (F) 19-06-1970 08-01-99 L-8 74300 08-01-99 9830402047 Bankra Birati, Group , Kolkata Kolkata-700051 T/33, Panchasayar, P.O. Garia, Eastern Printing Estt. & Accounts 5 Group 'B' Shri B.R. Chakraborty P.R. Chakraborty(F) 07-02-57 02-04-84 L-7 58600 02-08-17 9433621880 Kolkata-700084 Group , Kolkata Officer Namita Apartment, Eastern Printing 6 Survey Assistant B(Non-Gz) Shri Amit Kumar Biswas A.K. -

Notification No. 2369 RG Dated, Calcutta, the 18Th May, 2018
HIGH COURT, CALCUTTA APPELLATE SIDE N O T I F I C A T I O N No.2369 RG Dated, Calcutta, the 18th May, 2018 th In terms of the Advertisement No. 1654-RG dated 16 April, 2018, three (3) lists are published, List-A, List-B and List-C, for appearing in the th preliminary test to be held at 11:00 A.M. on 11 June, 2018 at Public Service Commission Office, 161A Shyama Prasad Mukherjee Road, Kolkata- 700 026, for Direct Recruitment from the Members of the Bar in the cadre of District Judge (Entry Level) in the West Bengal Judicial Service. List- A contains the names of the candidates whose applications are “in order” and they are provisionally declared eligible to appear in the said examination, subject to further verification of their eligibility. List-B contains the names of the candidates whose applications are “disqualified” for the reason(s) mentioned therein. List-C (Warning List) contains the names of the candidates whose applications are “defective” for the reasons mentioned in the column “Defects”. The candidates whose names are appearing in the List-C (Warning List) are directed to cure the defect(s) of their applications by providing the required document(s) as demanded in the Notification No. 1654 –RG dated 16.04.2018 on 24.05.2018 (Thursday) and 25.05.2018 (Friday) from 12 noon th to 4:30 p.m. in the Recruitment Cell, 6 Floor, Room No. 654, Sesquicentenary Building, High Court, Calcutta. On receipt of satisfactory required document(s) within the stipulated time mentioned above from the candidates mentioned in the List- “C” (Warning List), they will be provisionally allowed to appear at the above mentioned Examination subject to verification of eligibility by the authority.