Download E-JRS Copy
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Paralaurionite Pbcl(OH) C 2001-2005 Mineral Data Publishing, Version 1
Paralaurionite PbCl(OH) c 2001-2005 Mineral Data Publishing, version 1 Crystal Data: Monoclinic. Point Group: 2/m. Crystals are thin to thick tabular k{100}, or elongated along [001], to 3 cm; {100} is usually dominant, but may show many other forms. Twinning: Almost all crystals are twinned by contact on {100}. Physical Properties: Cleavage: {001}, perfect. Tenacity: Flexible, due to twin gliding, but not elastic. Hardness = Soft. D(meas.) = 6.05–6.15 D(calc.) = 6.28 Optical Properties: Transparent to translucent. Color: Colorless, white, pale greenish, yellowish, yellow-orange, rarely violet; colorless in transmitted light. Luster: Subadamantine. Optical Class: Biaxial (–). Pleochroism: Noted in violet material. Orientation: Y = b; Z ∧ c =25◦. Dispersion: r< v,strong. Absorption: Y > X = Z. α = 2.05(1) β = 2.15(1) γ = 2.20(1) 2V(meas.) = Medium to large. Cell Data: Space Group: C2/m. a = 10.865(4) b = 4.006(2) c = 7.233(3) β = 117.24(4)◦ Z=4 X-ray Powder Pattern: Laurium, Greece. 5.14 (10), 3.21 (10), 2.51 (9), 2.98 (7), 3.49 (6), 2.44 (6), 2.01 (6) Chemistry: (1) (2) (3) Pb 78.1 77.75 79.80 O [3.6] 6.00 3.08 Cl 14.9 12.84 13.65 H2O 3.4 3.51 3.47 insol. 0.09 Total [100.0] 100.19 100.00 (1) Laurium, Greece. (2) Tiger, Arizona, USA. (3) PbCl(OH). Polymorphism & Series: Dimorphous with laurionite. Occurrence: A secondary mineral formed through alteration of lead-bearing slag by sea water or in hydrothermal polymetallic mineral deposits. -

Memorials of Old Wiltshire I
M-L Gc 942.3101 D84m 1304191 GENEALOGY COLLECTION I 3 1833 00676 4861 Digitized by tine Internet Arciiive in 2009 with funding from Allen County Public Library Genealogy Center http://www.archive.org/details/memorialsofoldwiOOdryd '^: Memorials OF Old Wiltshire I ^ .MEMORIALS DF OLD WILTSHIRE EDITED BY ALICE DRYDEN Editor of Meinoriah cf Old Northamptonshire ' With many Illustrations 1304191 PREFACE THE Series of the Memorials of the Counties of England is now so well known that a preface seems unnecessary to introduce the contributed papers, which have all been specially written for the book. It only remains for the Editor to gratefully thank the contributors for their most kind and voluntary assistance. Her thanks are also due to Lady Antrobus for kindly lending some blocks from her Guide to Amesbury and Stonekenge, and for allowing the reproduction of some of Miss C. Miles' unique photographs ; and to Mr. Sidney Brakspear, Mr. Britten, and Mr. Witcomb, for the loan of their photographs. Alice Dryden. CONTENTS Page Historic Wiltshire By M. Edwards I Three Notable Houses By J. Alfred Gotch, F.S.A., F.R.I.B.A. Prehistoric Circles By Sir Alexander Muir Mackenzie, Bart. 29 Lacock Abbey .... By the Rev. W. G. Clark- Maxwell, F.S.A. Lieut.-General Pitt-Rivers . By H. St. George Gray The Rising in the West, 1655 . The Royal Forests of Wiltshire and Cranborne Chase The Arundells of Wardour Salisbury PoHtics in the Reign of Queen Anne William Beckford of Fonthill Marlborough in Olden Times Malmesbury Literary Associations . Clarendon, the Historian . Salisbury .... CONTENTS Page Some Old Houses By the late Thomas Garner 197 Bradford-on-Avon By Alice Dryden 210 Ancient Barns in Wiltshire By Percy Mundy . -

Standard X-Ray Diffraction Powder Patterns
NBS MONOGRAPH 25 — SECTION 1 Standard X-ray Diffraction U.S. DEPARTMENT OF COMMERCE NATIONAL BUREAU OF STANDARDS THE NATIONAL BUREAU OF STANDARDS Functions and Activities The functions of the National Bureau of Standards are set forth in the Act of Congress, March 3, 1901, as amended by Congress in Public Law 619, 1950. These include the development and maintenance of the national standards of measurement and the provision of means and methods for making measurements consistent with these standards; the determination of physical constants and properties of materials; the development of methods and instruments for testing materials, devices, and structures; advisory services to government agencies on scien- tific and technical problems; invention and development of devices to serve special needs of the Government; and the development of standard practices, codes, and specifications. The work includes basic and applied research, development, engineering, instrumentation, testing, evaluation, calibration services, and various consultation and information services. Research projects are also performed for other government agencies when the work relates to and supplements the basic program of the Bureau or when the Bureau's unique competence is required. The scope of activities is suggested by the listing of divisions and sections on the inside of the back cover. Publications The results of the Bureau's research are published either in the Bureau's own series of publications or in the journals of professional and scientific societies. The Bureau itself publishes three periodicals available from the Government Printing Office: The Journal of Research, published in four separate sections, presents complete scientific and technical papers; the Technical News Bulletin presents summary and preliminary reports on work in progress; and Basic Radio Propagation Predictions provides data for determining the best frequencies to use for radio communications throughout the world. -

Cumberland and Westmorland Herald Index of Soldiers 1914-1919
Cumberland and Westmorland Herald Index of soldiers 1914-1919 Page and Service Colu Surname Forename Rank Age Regiment No. Portrait Address Date and Place Reason Date mn Extra Information Abbott Allan Private Middlesex Keswick 30/11/1917 Killed 29/12/1917 1F article; obituary 5G Abbott Henry Private Border Regiment Alston Died of wounds 29/07/1916 1e Photograph 05/08/1916 3d Abbott John Sgt-Major Norfolk Penrith 12/11/1916 Killed 06/01/1917 1E article Abbott W Private 18 Machine Gun Corps Lazonby 29/09/1918 Died 12/10/1918 1E from wounds: article Abott Hugh Private 34 Canadians Lazonby 04/04/1918 Died 20/04/1918 3G from wounds: article : obituary 5F Abraham J C Lieutenant Keswick Dispatches 16/03/1918 6C " For meritorious service in the field " Adam Charles J Private 28 Winnipeg Cameron High No Winnipeg Canada 23/04/1915 Missing 22/05/1915 1f Originally from Castlegate, PH. Confirmed Killed in edition 28/08/1915 p5h Adamthwaite John Private Royal Field Artillery Isle of Wreay 11/05/1917 Killed 05/05/1917 1C article Adamthwaite Private Yes Bolton le Sands Killed 12/05/1917 1E Addison Walter J Private Canadians Pooley Bridge Wounded 20/10/1917 1D Airey Frank Private Border Regiment Yes Threlkeld 10/04/1918 PoW 08/06/1918 1D article 3D Airey Harvey Corporal Yes Shap Distinguished Conduct Medal 18/05/1918 3C no details Airey Norman Private Shap Wounded 22/06/1918 1E Alcock Robert Private Hatcliffe Bridge PoW 01/09/1917 3E previously reported Missing Alderson C R 2nd Lieutenant R E Yes Penrith Military Cross 01/12/1917 5F article :also Military -

Mineral Processing
Mineral Processing Foundations of theory and practice of minerallurgy 1st English edition JAN DRZYMALA, C. Eng., Ph.D., D.Sc. Member of the Polish Mineral Processing Society Wroclaw University of Technology 2007 Translation: J. Drzymala, A. Swatek Reviewer: A. Luszczkiewicz Published as supplied by the author ©Copyright by Jan Drzymala, Wroclaw 2007 Computer typesetting: Danuta Szyszka Cover design: Danuta Szyszka Cover photo: Sebastian Bożek Oficyna Wydawnicza Politechniki Wrocławskiej Wybrzeze Wyspianskiego 27 50-370 Wroclaw Any part of this publication can be used in any form by any means provided that the usage is acknowledged by the citation: Drzymala, J., Mineral Processing, Foundations of theory and practice of minerallurgy, Oficyna Wydawnicza PWr., 2007, www.ig.pwr.wroc.pl/minproc ISBN 978-83-7493-362-9 Contents Introduction ....................................................................................................................9 Part I Introduction to mineral processing .....................................................................13 1. From the Big Bang to mineral processing................................................................14 1.1. The formation of matter ...................................................................................14 1.2. Elementary particles.........................................................................................16 1.3. Molecules .........................................................................................................18 1.4. Solids................................................................................................................19 -
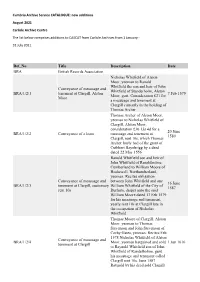
New Additions to CASCAT from Carlisle Archives
Cumbria Archive Service CATALOGUE: new additions August 2021 Carlisle Archive Centre The list below comprises additions to CASCAT from Carlisle Archives from 1 January - 31 July 2021. Ref_No Title Description Date BRA British Records Association Nicholas Whitfield of Alston Moor, yeoman to Ranald Whitfield the son and heir of John Conveyance of messuage and Whitfield of Standerholm, Alston BRA/1/2/1 tenement at Clargill, Alston 7 Feb 1579 Moor, gent. Consideration £21 for Moor a messuage and tenement at Clargill currently in the holding of Thomas Archer Thomas Archer of Alston Moor, yeoman to Nicholas Whitfield of Clargill, Alston Moor, consideration £36 13s 4d for a 20 June BRA/1/2/2 Conveyance of a lease messuage and tenement at 1580 Clargill, rent 10s, which Thomas Archer lately had of the grant of Cuthbert Baynbrigg by a deed dated 22 May 1556 Ranold Whitfield son and heir of John Whitfield of Ranaldholme, Cumberland to William Moore of Heshewell, Northumberland, yeoman. Recites obligation Conveyance of messuage and between John Whitfield and one 16 June BRA/1/2/3 tenement at Clargill, customary William Whitfield of the City of 1587 rent 10s Durham, draper unto the said William Moore dated 13 Feb 1579 for his messuage and tenement, yearly rent 10s at Clargill late in the occupation of Nicholas Whitfield Thomas Moore of Clargill, Alston Moor, yeoman to Thomas Stevenson and John Stevenson of Corby Gates, yeoman. Recites Feb 1578 Nicholas Whitfield of Alston Conveyance of messuage and BRA/1/2/4 Moor, yeoman bargained and sold 1 Jun 1616 tenement at Clargill to Raynold Whitfield son of John Whitfield of Randelholme, gent. -

National Sample from the 1851 Census of Great Britain List of Sample Clusters
NATIONAL SAMPLE FROM THE 1851 CENSUS OF GREAT BRITAIN LIST OF SAMPLE CLUSTERS The listing is arranged in four columns, and is listed in cluster code order, but other orderings are available. The first column gives the county code; this code corresponds with the county code used in the standardised version of the data. An index of the county codes forms Appendix 1 The second column gives the cluster type. These cluster types correspond with the stratification parameter used in sampling and have been listed in Background Paper II. Their definitions are as follows: 11 English category I 'Communities' under 2,000 population 12 Scottish category I 'Communities' under 2,000 population 21 Category IIA and VI 'Towns' and Municipal Boroughs 26 Category IIB Parliamentary Boroughs 31 Category III 'Large non-urban communities' 41 Category IV Residual 'non-urban' areas 51 Category VII Unallocable 'urban' areas 91 Category IX Institutions The third column gives the cluster code numbers. This corresponds to the computing data set name, except that in the computing data set names the code number is preceded by the letters PAR (e.g. PAR0601). The fourth column gives the name of the cluster community. It should be noted that, with the exception of clusters coded 11,12 and 91, the cluster unit is the enumeration district and not the whole community. Clusters coded 11 and 12, however, correspond to total 'communities' (see Background Paper II). Clusters coded 91 comprise twenty successive individuals in every thousand, from a list of all inmates of institutions concatenated into a continuous sampling frame; except that 'families' are not broken, and where the twenty individuals come from more than one institution, each institution forms a separate cluster. -

Macphersonite Pb4(SO4)(CO3)2(OH)2 C 2001-2005 Mineral Data Publishing, Version 1
Macphersonite Pb4(SO4)(CO3)2(OH)2 c 2001-2005 Mineral Data Publishing, version 1 Crystal Data: Orthorhombic, pseudohexagonal. Point Group: 2/m 2/m 2/m. Crystals are commonly pseudohexagonal, thin to tabular on {010}, to 1 cm. Twinning: Common, lamellar and contact, composition plane {102}. Physical Properties: Cleavage: On {010}, perfect. Fracture: Uneven. Hardness = 2.5–3 D(meas.) = 6.50–6.55 D(calc.) = 6.60–6.65 May exhibit a bright yellow fluorescence under SW and LW UV. Optical Properties: Semitransparent. Color: Colorless, white, very pale amber. Luster: Adamantine to resinous. Optical Class: Biaxial (–). Orientation: X = b; Y = c; Z = a. Dispersion: r> v,moderate. α = 1.87 β = 2.00 γ = 2.01 2V(meas.) = 35◦–36◦ Cell Data: Space Group: P cab. a = 10.383(2) b = 23.050(5) c = 9.242(2) Z = 8 X-ray Powder Pattern: Argentolle mine, France; may show preferred orientation. 3.234 (100), 2.654 (90), 3.274 (50), 2.598 (30), 2.310 (30), 2.182 (30), 2.033 (30) Chemistry: (1) (2) (3) SO3 6.6 7.65 7.42 CO2 8.8 8.47 8.16 CuO 0.1 CdO 0.1 PbO 83.4 83.59 82.75 + H2O 1.3 1.93 1.67 Total 100.3 101.64 100.00 (1) Leadhills, Scotland; by electron microprobe, average of ten analyses, CO2 by evolved gas analysis, H2O by TGA; corresponds to (Pb4.08Cu0.10Cd0.07)Σ=4.25(S0.90O4)(C1.09O3)2(OH)1.58. (2) Argentolle mine, France; corresponds to Pb4.06(S1.03O4)(C1.04O3)2(OH)2.32. -

A Biographical Dictionary of Nineteenth Century Antique and Curiosity Dealers
This is a repository copy of A Biographical Dictionary of Nineteenth Century Antique and Curiosity Dealers. White Rose Research Online URL for this paper: http://eprints.whiterose.ac.uk/42902/ Book: Westgarth, MW (2009) A Biographical Dictionary of Nineteenth Century Antique and Curiosity Dealers. Regional Furniture, XXIII . Regional Furniture Society , Glasgow . Reuse Unless indicated otherwise, fulltext items are protected by copyright with all rights reserved. The copyright exception in section 29 of the Copyright, Designs and Patents Act 1988 allows the making of a single copy solely for the purpose of non-commercial research or private study within the limits of fair dealing. The publisher or other rights-holder may allow further reproduction and re-use of this version - refer to the White Rose Research Online record for this item. Where records identify the publisher as the copyright holder, users can verify any specific terms of use on the publisher’s website. Takedown If you consider content in White Rose Research Online to be in breach of UK law, please notify us by emailing [email protected] including the URL of the record and the reason for the withdrawal request. [email protected] https://eprints.whiterose.ac.uk/ promoting access to White Rose research papers Universities of Leeds, Sheffield and York http://eprints.whiterose.ac.uk/ White Rose Research Online URL for this paper: http://eprints.whiterose.ac.uk/42902/ Published book: Westgarth, MW (2009) A Biographical Dictionary of Nineteenth Century Antique and Curiosity Dealers. Regional Furniture, XXIII . Regional Furniture Society White Rose Research Online [email protected] 148132:97095_book 6/4/10 10:11 Page cov1 REGIONAL FURNITURE 2009 148132:97095_book 6/4/10 10:11 Page cov2 THE REGIONAL FURNITURE SOCIETY FOUNDED 1984 Victor Chinnery President Michael Legg Vice President COUNCIL David Dewing Chairman Alison Lee Hon. -

Rulers of Opinion Women at the Royal Institution of Great Britain, 1799
Rulers of Opinion Women at the Royal Institution of Great Britain, 1799-1812 Harriet Olivia Lloyd UCL Submitted for the Degree of Doctor of Philosophy in History of Science 2018 1 I, Harriet Olivia Lloyd, confirm that the work presented in this thesis is my own. Where information has been derived from other sources, I confirm that this has been indicated in the thesis. 2 Abstract This thesis examines the role of women at the Royal Institution of Great Britain in its first decade and contributes to the field by writing more women into the history of science. Using the method of prosopography, 844 women have been identified as subscribers to the Royal Institution from its founding on 7 March 1799, until 10 April 1812, the date of the last lecture given by the chemist Humphry Davy (1778- 1829). Evidence suggests that around half of Davy’s audience at the Royal Institution were women from the upper and middle classes. This female audience was gathered by the Royal Institution’s distinguished patronesses, who included Mary Mee, Viscountess Palmerston (1752-1805) and the chemist Elizabeth Anne, Lady Hippisley (1762/3-1843). A further original contribution of this thesis is to explain why women subscribed to the Royal Institution from the audience perspective. First, Linda Colley’s concept of the “service élite” is used to explain why an institution that aimed to apply science to the “common purposes of life” appealed to fashionable women like the distinguished patronesses. These women were “rulers of opinion,” women who could influence their peers and transform the image of a degenerate ruling class to that of an élite that served the nation. -

RESEARCH FRAMEWORK 100 the Derwent Valley 100 95 95
DERWENT VALLEY MILLS DERWENT VALLEY 100 The Derwent Valley 100 95 95 75 The Valley that changed the World 75 25 DERWENT VALLEY MILLS WORLD HERITAGE SITE 25 5 RESEARCH FRAMEWORK 5 0 0 Edited by David Knight Inscriptions on UNESCO's SITE RESEARCH FRAMEWORK WORLD HERITAGE prestigious World Heritage List are based on detailed research into the sites' evolution and histories. The role of research does not end with the presentation of the nomination or indeed the inscription itself, which is rst and foremost a starting point. UNESCO believes that continuing research is also central to the preservation and interpretation of all such sites. I therefore wholeheartedly welcome the publication of this document, which will act as a springboard for future investigation. Dr Mechtild Rössler, Director of the UNESCO Division for Heritage and the UNESCO World Heritage Centre 100 100 95 95 75 75 ONIO MU IM N R D T IA A L P W L O A I 25 R 25 D L D N H O E M R E I T I N A O GE IM 5 PATR 5 United Nations Derwent Valley Mills Educational, Scientific and inscribed on the World 0 Cultural Organisation Heritage List in 2001 0 Designed and produced by Derbyshire County Council, County Hall, Matlock Derbyshire DE4 3AG Research Framework cover spread print 17 August 2016 14:18:36 100 100 95 95 DERWENT VALLEY MILLS WORLD HERITAGE SITE 75 75 RESEARCH FRAMEWORK 25 25 5 Edited by David Knight 5 0 0 Watercolour of Cromford, looking upstream from the bridge across the River Derwent, painted by William Day in 1789. -

Standard X-Ray Diffraction Powder Patterns
NBS MONOGRAPH 25—SECTION 4 Standard X-ray Diffraction Powder Patterns U.S. DEPARTMENT OF COMMERCE NATIONAL BUREAU OF STANDARDS THE NATIONAL BUREAU OF STANDARDS The National Bureau of Standards is a principal focal point in the Federal Government for assuring maximum application of the physical and engineering sciences to the advancement of technology in industry and commerce. Its responsibilities include development and mainte- nance of the national standards of measurement, and the provisions of means for making measurements consistent with those standards; determination of physical constants and properties of materials; development of methods for testing materials, mechanisms, and structures, and making such tests as may be necessary, particularly for government agencies; cooperation in the establishment of standard practices for incorporation in codes and specifi- cations advisory service to government agencies on scientific and technical problems ; invention ; and development of devices to serve special needs of the Government; assistance to industry, business, and consumers m the development and acceptance of commercial standards and simplified trade practice recommendations; administration of programs in cooperation with United States business groups and standards organizations for the development of international standards of practice; and maintenance of a clearinghouse for the collection and dissemination of scientific, technical, and engineering information. The scope of the Bureau's activities is suggested in the following listing of its three Institutes and their organizatonal units. Institute for Basic Standards. Applied Mathematics. Electricity. Metrology. Mechanics. Heat. Atomic Physics. Physical Chemistry. Laboratory Astrophysics.* Radiation Phys- ics. Radio Standards Laboratory:* Radio Standards Physics; Radio Standards Engineering. Office of Standard Reference Data. Institute for Materials Research.