Analysis of Evoked Activity Patterns of Human Thalamic Ventrolateral Neurons During Verbally Ordered Voluntary Movements
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Neurophysiological Characterisation of Neurons in the Rostral Nucleus Reuniens in Health and Disease
Neurophysiological characterisation of neurons in the rostral nucleus reuniens in health and disease. Submitted by Darren Walsh, to the University of Exeter as a thesis for the degree of Doctor of Philosophy in Medical Studies, September 2017. This thesis is available for Library use on the understanding that it is copyright material and that no quotation from the thesis may be published without proper acknowledgement. I certify that all material in this thesis which is not my own work has been identified and that no material has previously been submitted and approved for the award of a degree by this or any other University. (Signature) ……………………………………………………………………………… Word Count = 44,836 1 Abstract Evidence is mounting for a role of the nucleus reuniens (Re) in higher cognitive function. Despite growing interest, very little is known about the intrinsic neurophysiological properties of Re neurons and, to date, no studies have examined if alterations to Re neurons may contribute to cognitive deficits associated with normal aging or dementia. Work presented chapter 3 provides the first detailed description of the intrinsic electrophysiological properties of rostral Re neurons in young adult (~5 months) C57- Bl/6J mice. This includes a number of findings which are highly atypical for thalamic relay neurons including tonic firing in the theta frequency at rest, a paucity of hyperpolarisation-activated cyclic nucleotide–gated (HCN) mediated currents, and a diversity of responses observed in response to depolarising current injections. Additionally this chapter includes a description of a novel form of intrinsic plasticity which alters the functional output of Re neurons. Chapter 4 investigates whether the intrinsic properties of Re neurons are altered in aged (~15 month) C57-Bl/6J mice as compared to a younger control group (~5 months). -

Thalamus.Pdf
Thalamus 583 THALAMUS This lecture will focus on the thalamus, a subdivision of the diencephalon. The diencephalon can be divided into four areas, which are interposed between the brain stem and cerebral hemispheres. The four subdivisions include the hypothalamus to be discussed in a separate lecture, the ventral thalamus containing the subthalamic nucleus already discussed, the epithalamus which is made up mostly of the pineal body, and the dorsal thalamus (henceforth referred to as the thalamus) which is the focus of this lecture. Although we will not spend any time in lecture on the pineal body, part of the epithalamus, it does have some interesting features as well as some clinical relevance. The pineal is a small midline mass of glandular tissue that secretes the hormone melatonin. In lower mammals, melatonin plays a central role in control of diurnal rhythms (cycles in body states and hormone levels that follow the day- night cycle). In humans, at least a portion of the control of diurnal rhythms has been taken over by the hypothalamus, but there is increasing evidence that the pineal and melatonin play at least a limited role. Recent investigations have demonstrated a role for melatonin in sleep, tumor reduction and aging. Additionally, based on the observation that tumors of the pineal can induce a precocious puberty in males it has been suggested that the pineal is also involved in timing the onset of puberty. In many individuals the pineal is partially calcified and can serve as a marker for the midline of the brain on x- rays. Pathological processes can sometimes be detected by a shift in its position. -

Diencephalon Diencephalon
Diencephalon Diencephalon • Thalamus dorsal thalamus • Hypothalamus pituitary gland • Epithalamus habenular nucleus and commissure pineal gland • Subthalamus ventral thalamus subthalamic nucleus (STN) field of Forel Diencephalon dorsal surface Diencephalon ventral surface Diencephalon Medial Surface THALAMUS Function of the Thalamus • Sensory relay – ALL sensory information (except smell) • Motor integration – Input from cortex, cerebellum and basal ganglia • Arousal – Part of reticular activating system • Pain modulation – All nociceptive information • Memory & behavior – Lesions are disruptive Classification of Thalamic Nuclei I. Lateral Nuclear Group II. Medial Nuclear Group III. Anterior Nuclear Group IV. Posterior Nuclear Group V. Metathalamic Nuclear Group VI. Intralaminar Nuclear Group VII. Thalamic Reticular Nucleus Classification of Thalamic Nuclei LATERAL NUCLEAR GROUP Ventral Nuclear Group Ventral Posterior Nucleus (VP) ventral posterolateral nucleus (VPL) ventral posteromedial nucleus (VPM) Input to the Thalamus Sensory relay - Ventral posterior group all sensation from body and head, including pain Projections from the Thalamus Sensory relay Ventral posterior group all sensation from body and head, including pain LATERAL NUCLEAR GROUP Ventral Lateral Nucleus Ventral Anterior Nucleus Input to the Thalamus Motor control and integration Projections from the Thalamus Motor control and integration LATERAL NUCLEAR GROUP Prefrontal SMA MI, PM SI Ventral Nuclear Group SNr TTT GPi Cbll ML, STT Lateral Dorsal Nuclear Group Lateral -

Studies on Ti&Diencephalon of the Vir'i' 4Nia Opossum
STUDIES ON TI&DIENCEPHALON OF THE VIR'I' 4NIA OPOSSUM PART 11. THE FIB,,RiC70NNECTIONS IN NORMAL AND EXPP MENTAL MATERIAL' LAVID BODIAN National Research Coq :,'gFeJlow in Medicine, Laboratory of Comparative N6dro1ogyl Depart3 ,# of Anatomy, The University of Michigan, Ann Arbo., and the Department of Anatomy, Thq,gniueraity of Chicago, Illinois THIBTI-'*.INPLATES (TWENTY-SIX FIGURES) CONTENTS Introduction ..... .............................................. 208 Material and methods.. .................. ..................... 209 Internal capsule and tha. mie radiations ................................ 210 Dorsal thalamic eommisswes and internal medullary lamina. .............. 213 Supraoptic decussations ................................................ 215 Connections of the dorsal thalamus.. ................................... 223 Anterior nuclear group. ............... ........... 223 Medial nuclear group ............................................. 224 Midline and commissural nuclei. ... ............... 227 Lateral nuclear group ............................................. 230 Ventral nuclear group and medial lemniscus.. ........................ 231 Geniculate nuelel and associated optic and auditory nuclei of the di- mesencephal I: junction. Tecto-reuniens fiber systems. ............ 233 The optic tracts and nucleus opticus tegmenti.. ...................... 237 Conriections of the ventral thalamus. ....... ............... 239 Zona incerta .................................................. Nucleus genic! +us lateralis ventralis. -

The Diencephalon Is Located Near the Midline of the Brain Above the Midbrain
Neuroanatomy Dr. Maha ELBeltagy Assistant Professor of Anatomy Faculty of Medicine The University of Jordan 2018 10/15/17 Prof Yousry Diencephalon Diencephalon The Diencephalon is located near the midline of the brain above the midbrain. Developed from the fbiforebrain vesilicle (prosencephalon). More primitive than the cerebral cortex and lies under it. Surrounds the third ventricle The Diencephalon • The cavity of the 3rd ventricle divides the diencephalon into 2 halves. • Each half is divided by the hypothalamic sulcus (which extends from the interventricular foramen to the cerebral aqueduct) into ventral & dorsal parts: Dorsal part includes: ‐ Thalamus, Epithalamus & Matathalamus. Ventral part includes: ‐ Hypothalamus & Subthalamus Interventricular foramen Thalamus Hypothalamic sulcus Hypothalamus cerebral aqueduct THALAMUS THALAMUS • It is a large egg shaped mass of grey matter which forms the main sensory relay station for the cerebral cortex. Interthalamic • It forms part of the lateral wall adhesion of the 3rd ventricle & the part of the floor of the body of the lateral ventricle. • The 2 thalami are connected by interthalamic adhesion. THALAMUS Shape and rel ati ons: Oval shape has 2 ends and 4 surfaces: Anterior end: narrow and forms the posterior boundary of the IVF. Posterior end: Pulvinar overhanging the MGB and LGB. Upper surface : floor of body of lateral ventricle. Medial surface: lateral wall of third ventricle Lateral surface: caudate above &lentiform below separated from it by posterior limb of internal capsule Lower surface: hypothalamus anterior and subthalamus posterior Classification of Thalamic Nuclei I. Lateral Nuclear Group II. Medial Nuclear Group III. Anterior Nuclear Group IV. Posterior Nuclear Group V. MhliMetathalamic NlNuclear Group VI. -

System and Method for Neuroenhancement to Enhance Emotional
) ( (51) International Patent Classification: DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, HN, A61B 5/0484 (2006.01) A61B 5/16 (2006.01) HR, HU, ID, IL, IN, IR, IS, JO, JP, KE, KG, KH, KN, KP, KR, KW, KZ, LA, LC, LK, LR, LS, LU, LY, MA, MD, ME, (21) International Application Number: MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, PCT/US20 18/068220 OM, PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, (22) International Filing Date: SC, SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, 31 December 2018 (3 1. 12.2018) TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. (25) Filing Language: English (84) Designated States (unless otherwise indicated, for every kind of regional protection available) . ARIPO (BW, GH, (26) Publication Language: English GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, ST, SZ, TZ, (30) Priority Data: UG, ZM, ZW), Eurasian (AM, AZ, BY, KG, KZ, RU, TJ, 62/612,565 31 December 2017 (3 1. 12.2017) US TM), European (AL, AT, BE, BG, CH, CY, CZ, DE, DK, EE, ES, FI, FR, GB, GR, HR, HU, IE, IS, IT, LT, LU, LV, (71) Applicant: NEUROENHANCEMENT LAB, LLC MC, MK, MT, NL, NO, PL, PT, RO, RS, SE, SI, SK, SM, [US/US]; 75 Montebello Park, Suffern, New York 10901 TR), OAPI (BF, BJ, CF, CG, Cl, CM, GA, GN, GQ, GW, (US). KM, ML, MR, NE, SN, TD, TG). (72) Inventor; and (71) Applicant: POLTORAK, Alexander [US/US]; 128 W. -

The Neuroanatomical Connections and Somatotopic Organization of the Posterior Nuclear Complex of the Rat Thalamus
Loyola University Chicago Loyola eCommons Dissertations Theses and Dissertations 1985 The Neuroanatomical Connections and Somatotopic Organization of the Posterior Nuclear Complex of the Rat Thalamus E. Luke Bold Loyola University Chicago Follow this and additional works at: https://ecommons.luc.edu/luc_diss Part of the Anatomy Commons Recommended Citation Bold, E. Luke, "The Neuroanatomical Connections and Somatotopic Organization of the Posterior Nuclear Complex of the Rat Thalamus" (1985). Dissertations. 2306. https://ecommons.luc.edu/luc_diss/2306 This Dissertation is brought to you for free and open access by the Theses and Dissertations at Loyola eCommons. It has been accepted for inclusion in Dissertations by an authorized administrator of Loyola eCommons. For more information, please contact [email protected]. This work is licensed under a Creative Commons Attribution-Noncommercial-No Derivative Works 3.0 License. Copyright © 1985 E. Luke Bold THE NEUROANATOMICAL CONNECTIONS AND SOMATOTOPIC ORGANIZATION OF THE POSTERIOR NUCLEAR COMPLEX OF THE RAT THALAMUS by E. LUKE/BOLp A DISSERTATION SUBMITTED TO THE FACULTY OF THE GRADUATE SCHOOL OF LOYOLA UNIVERSITY OF CHICAGO IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY MARCH 1985 DEDICATION TO MY FAMILY ii ACKNOWLEDGEMENTS I would like to thank my advisor, Dr. E.J. Neafsey, for his patience, support, encouragement and unfailing guidance which made this work possible. Thanks are also due to the other members of the dissertation committee, Dr. A.J. Castro, Dr. T.s. Gray, Dr. C.J. Robinson, Dr. R.D. Wurster, and Dr. W.I. Welker, who collectively provided insightful and helpful comments to improve the manuscript. -

A Comparison of Thalamic Afferents to the Rostral and Caudal Forelimb Regions of Rat Motor Cortex: an Experimental Investigation Using Horseradish Peroxidase
Loyola University Chicago Loyola eCommons Master's Theses Theses and Dissertations 1982 A Comparison of Thalamic Afferents to the Rostral and Caudal Forelimb Regions of Rat Motor Cortex: An Experimental Investigation Using Horseradish Peroxidase E. Luke Bold Loyola University Chicago Follow this and additional works at: https://ecommons.luc.edu/luc_theses Part of the Anatomy Commons Recommended Citation Bold, E. Luke, "A Comparison of Thalamic Afferents to the Rostral and Caudal Forelimb Regions of Rat Motor Cortex: An Experimental Investigation Using Horseradish Peroxidase" (1982). Master's Theses. 3171. https://ecommons.luc.edu/luc_theses/3171 This Thesis is brought to you for free and open access by the Theses and Dissertations at Loyola eCommons. It has been accepted for inclusion in Master's Theses by an authorized administrator of Loyola eCommons. For more information, please contact [email protected]. This work is licensed under a Creative Commons Attribution-Noncommercial-No Derivative Works 3.0 License. Copyright © 1982 E. Luke Bold A COMPARISON OF THALAMIC AFFERENTS TO THE ROSTRAL AND CAUDAL FORELIMB REGIONS OF RAT MOTOR CORTEX AN EXPERIMENTAL INVESTIGATION USING HORSERADISH PEROXIDASE by E. LUKE BOLD A THESIS SUBMITTED TO THE GRADUATE SCHOOL OF LOYOLA UNIVERSITY OF CHICAGO IN PARTIAL FULLFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF MASTER OF SCIENCE APRIL 1982 ACKNOWLEDGEMENTS The author wishes to acknowledge the patience and unfailing guidance of his advisor, Dr. E.J. Neafsey, for his continued support throughout the extent of this project. Also thanks to the other members of the thesis committee, Dr. R.D. Wurster, Dr. A.J. Castro, and Dr. -
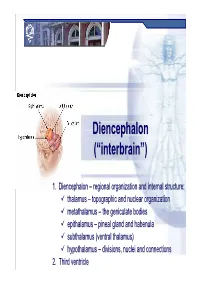
Diencephalondiencephalon ((““Interbraininterbrain ””))
DiencephalonDiencephalon ((““interbraininterbrain ””)) 1. Diencephalon – regional organization and internal structure: thalamus – topographic and nuclear organization metathalamus – the geniculate bodies epithalamus – pineal gland and habenula subthalamus (ventral thalamus) hypothalamus – divisions, nuclei and connections 2. Third ventricle Diencephalon EmbryologicEmbryologic developmentdevelopment Embryonic origin:origin side walls of the prosencephalon (forebrain) Location – at the midline of the brain: caudally – mesencephalon cranially – telencephalon Functions: relay system between sensory input neurons and other parts of the brain works in tandem with the limbic system Prof. Dr. Nikolai Lazarov 2 Diencephalon DiencephalonDiencephalon –– grossgross structurestructure andand partsparts Prof. Dr. Nikolai Lazarov 3 Thalamus ThalamusThalamus –– externalexternal featuresfeatures two egg -shaped lobes of grey matter Gr. θάλαµος = room , chamber third ventricle medially Gr. θάλαµος = room , chamber hypothalamus hypothalamic sulcus Thalamus dorsalis : nuclear complex – 2% of the total brain rostral pole = tuberculum anterius nuclear complex – 2% of the total brain thalami about 80% caudal pole = pulvinar thalami of diencephalic mass (“cushioned seat ”) ~30 mm long interthalamic adhesion ~20 mm wide lamina affixa ~20 mm tall stria terminalis thalami Prof. Dr. Nikolai Lazarov 4 Thalamus ThalamusThalamus –– internalinternal structurestructure internal medullary lamina three major nuclear masses: (medial) – Y-shaped -

The Nuclear Pattern of the Non-Tectal Portions of the Midbrain and Isthmus in Ungtjlates
THE NUCLEAR PATTERN OF THE NON-TECTAL PORTIONS OF THE MIDBRAIN AND ISTHMUS IN UNGTJLATES LOIS A. GILLILAN Dr. Louis Merwin Gelston Fellow, Mrdical School, Universzty of Mickigan, Ann Arbor Department of Anatomy, University of Ptttsburgh, Pennsylvania TWENTY-SIX PLATES (TWENTY-SIX FIGURES) INTRODUCTION The material used for this study of the midbrain of the 16 cin pig (Sus scrofa), the adult sheep (Ovis aries) and the old horse (Equus caballus) consists of serially cut, transverse, toluidin blue sections. For the generous grant which made possible this research the writer wishes to express grateful acknowlcdgmeiit to the Horace H. Rackham School of Graduate Studies of the University of Michigan. Very little literature dealing with the ungulate midbrain has come to our attention. Papers by Solnitzsky ( '38 and '39) deal with the dorsal thalamic, subthalamic, and hypothalamic regions of the pig brain; they contain accounts of the pre- tectal regions, substantia nigra and the rostra1 tip of the red nucleus. Rose ('42 b) has found that the pretectal nucleus in the sheep is represented by two groups of cells. Le Gros Clark ( '26) observed that the Edinger-Westphal nucleus in the sheep was little differentiated, and in the pig th'e cells were only slightly different from the small medial raphe cells with which they become continuous anteriorly. The Edinger-Westphal nucleus in the pig is mostly a midline structure with only occasional bilateral clumping. Tsuchida ( '06) found no Edinger-Westphal nucleus in the sheep and in the horse a few scattered cells only. He observed no nucleus medianns anterior and no true nucleus of Perlia; in the horse no nucleus of 289 290 G. -

The Human Central Nervous System R
R. Nieuwenhuys J. Voogd C. van Huijzen The Human Central Nervous System R. Nieuwenhuys J. Voogd C. van Huijzen The Human Central Nervous System Fourth Edition With 391 Figures 12 Rudolf Nieuwenhuys M.D., Ph.D. Professor emeritus of Neuroanatomy The Netherlands Institute (home) for Neuroscience Papehof 25 Meibergdreef 47 1391 BD Abcoude 1105 BA Amsterdam The Netherlands The Netherlands [email protected] Jan Voogd M.D., Ph.D. Professor emeritus of Anatomy Department of Neuroscience (home) Erasmus University Rotterdam Rhijngeesterstraatweg 1 P.O. Box 2040 2342 AN Oegstgeest 3000 CA Rotterdam The Netherlands The Netherlands [email protected] Christiaan van Huijzen F.M.A.A. Medical Artist (retired) (home) Willem Degenstraat 2 6525 BW Nijmegen The Netherlands Library of Congress Control Number: 2007926177 ISBN 978-3-540-34684-5 Springer-Verlag Berlin Heidelberg New York ISBN 3-540-13441-7 3. Auflage Springer-Verlag Berlin Heidelberg New York This work is subject to copyright. All rights are reserved, whether the whole or part of the material is con- cerned, specifically the rights of translation, reprinting, reuse of illustrations, recitation, broadcasting, repro- duction on microfilm or in any other way, and storage in data banks. Duplication of this publication or parts thereof is permitted only under the provisions of the German Copyright Law of September 9, 1965, in its current version, and permission for use must always be obtained from Springer. Violations are liable for pro- secution under the German Copyright Law. Springer is a part of Springer Science+Business Media springer.com © Springer Berlin Heidelberg 1978, 1981, 1988, 2008 Printed in Germany The use of general descriptive names, registered names, trademarks, etc. -
The Interpretation Ofthe Degenerative Changes In
J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp.30.2.140 on 1 April 1967. Downloaded from J. Neurol. Neurosurg. Psychiat., 1967, 30, 140 The interpretation of the degenerative changes in the intralaminar nuclei of the thalamus T. P. S. POWELL AND W. M. COWAN From the University of Oxford, Department of Human Anatomy, South Parks Road, Oxford Although the intralaminar nuclei of the thalamus rostral part of the corpus callosum. In three brains are now known to have an organized, extrathalamic stereotactic lesions had been placed in the thalamus for projection through the internal capsule (Droogleever another investigation, and the remaining brains had and lesions in various parts of the cerebral cortex. All the Fortuyn, and Stefens, 1951; Nashold, Hanbery, animals with striatal lesions and four with cortical Olszewski, 1955; Powell and Cowan, 1956), the ablations were allowed to survive for periods ranging precise mode of termination of the efferents from between four and 18 weeks. These brains were fixed either these nuclei remains problematical. The experimental in 70% alcohol and 2% acetic acid or in 10% formol- evidence bearing upon the projection of these nuclei saline, and a regular one in five or one in 10 series of which is available at present permits of two possible sections throughout most of thc cerebral hemisphere was interpretations. Because the cells of these nuclei mounted and stained with thionine. The remainingProtected by copyright. undergo a certain degree of cellular degeneration as animals with cortical lesions survived for between one the result of large lesions of the cerebral cortex it and two weeks, were perfused with 10% formol-saline, that the intralaminar nuclei send and after a further period of fixation were sectioned on a has been suggested freezing microtome and stained according to the method their axons, or axon collaterals, directly to the of Nauta (1957).