Land Snail Communities Respond to Control of Invasive Rats in New Zealand Forests
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Husbandry of the Carnivorous Land Snail, Powelliphanta Augusta (Gastropoda: Pulmonata: Rhytdidae)
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by ResearchArchive at Victoria University of Wellington Husbandry of the Carnivorous Land Snail, Powelliphanta augusta (Gastropoda: Pulmonata: Rhytdidae) By Thomas Edward Allan A thesis submitted to the Victoria University of Wellington in fulfillment of the requirements for the degree of Master of Science in Ecological Restoration Victoria University of Wellington 2010 1 Abstract Key aspects of the captive husbandry of Powelliphanta augusta, a newly-described New Zealand land snail are investigated: how they should be managed and fed to provide individuals for release, and how a long-term captive population can be maintained as an insurance against extinction in the wild. This project arises from almost all members of this species having been brought into captivity due to their displacement in the wild by an opencast coalmine. Powelliphanta (F: Rhytididae) is a genus of endemic carnivorous snails, which includes 10 species, 27 subspecies and numerous undescribed taxa. As well as its diversity, Powelliphanta is renowned for the large size of its members (up to 90mm diameter) and their attractively-patterned shells. Most taxa are threatened due to habitat loss and predation by introduced mammalian predators. The study commences with a literature review to refine husbandry methods and to assess requirements for captive breeding of snails. From this review investigations are made into stocking densities, substrate, reproductive biology, body condition and growth of the P. augusta captive population. To determine an appropriate stocking density for P. augusta groups of six snails were kept at two densities; with either 720cm2, or 1440cm2 per group. -

The Impact of Land-Use Intensification on the Conservation Management of Native Forest Remnants Embedded Within Production Landscapes
The impact of land-use intensification on the conservation management of native forest remnants embedded within production landscapes Lisa H. Denmead B. Sc. (Biology), University of Canterbury, New Zealand This thesis is presented for the degree of Masters of Science by Research in Ecology School of Animal Biology The University of Western Australia 2012 Declaration I declare that this thesis is my own account of my research conducted during my period of enrolment at the University of Western Australia for the degree of Master of Science by Research. It has not previously been submitted for a degree at this or any other university. As stated in my Acknowledgements, my research has been assisted by interactions with a number of people, however any work that was shared with supervisors or other collaborators is mentioned below: Chapter Two: The research completed in this chapter was carried out as part of the larger research project - Riches to Rags: does elevated productivity drive ecosystem decay in adjacent natural habitats. I was fully involved in developing the study design for this project, which I used to answer the questions addressed in chapter two. Collaborators on this project include Raphael Didham, Elizabeth Deakin, Gary Barker, Jason Tylianakis and Louis Schipper. All the field and lab work for this chapter was carried out alongside my fellow student on the project, Elizabeth Deakin. I developed the research questions, carried out the analysis and wrote the chapter with the support of my supervisors Raphael Didham, Rachel Standish and Gary Barker. Chapter Three: The experiment in this chapter was conducted in one of the forest remnants used in the wider project but was separate from the main aims of the project. -

Evaluating the Effects of Biogeography and Fragmentation on The
Evaluating the effects of biogeography and fragmentation on the taxonomic, functional, and genetic diversity of forest-utilising bats in a South African biodiversity hotspot by Monika Ilka Moir Dissertation presented for the degree of Doctor of Philosophy in the Faculty of Science at Stellenbosch University Supervisors: Dr. Ramugondo V. Rambau Prof. Michael I. Cherry Dr. Leigh R. Richards December 2020 i Stellenbosch University https://scholar.sun.ac.za Declaration By submitting this dissertation electronically, I declare that the entirety of the work contained therein is my own, original work, that I am the sole author thereof (save to the extent explicitly otherwise stated), that reproduction and publication thereof by Stellenbosch University will not infringe any third party rights, and that I have not previously in its entirety or in part submitted it for obtaining any qualification. This dissertation includes one original paper published in a peer-reviewed journal with me as lead author, and three articles submitted and under peer-review. The development and writing of the papers (published and unpublished) were the principal responsibility of myself. Monika Ilka Moir August 2020 Copyright © 2020 Stellenbosch University All rights reserved ii Stellenbosch University https://scholar.sun.ac.za Abstract Bats are a highly diverse mammalian order and are some of the most economically important non-domesticated vertebrates, providing many ecosystem services that contribute to the global economy. Yet, they remain a largely understudied taxon, particularly in the Eastern Cape province of South Africa, in which basic surveys of bat assemblages utilising indigenous forests are lacking. Indigenous forests constitute South Africa’s smallest and most fragmented biome yet support disproportionally high biodiversity. -

Zoogeography of the Land and Fresh-Water Mollusca of the New Hebrides"
Web Moving Images Texts Audio Software Patron Info About IA Projects Home American Libraries | Canadian Libraries | Universal Library | Community Texts | Project Gutenberg | Children's Library | Biodiversity Heritage Library | Additional Collections Search: Texts Advanced Search Anonymous User (login or join us) Upload See other formats Full text of "Zoogeography of the land and fresh-water mollusca of the New Hebrides" LI E) RARY OF THE UNIVLRSITY Of ILLINOIS 590.5 FI V.43 cop. 3 NATURAL ri'^^OHY SURVEY. Zoogeography of the LAND AND FRESH-WATER MOLLUSCA OF THE New Hebrides ALAN SOLEM Curator, Division of Lower Invertebrates FIELDIANA: ZOOLOGY VOLUME 43, NUMBER 2 Published by CHICAGO NATURAL HISTORY MUSEUM OCTOBER 19, 1959 Library of Congress Catalog Card Number: 59-13761t PRINTED IN THE UNITED STATES OF AMERICA BY CHICAGO NATURAL HISTORY MUSEUM PRESS CONTENTS PAGE List of Illustrations 243 Introduction 245 Geology and Zoogeography 247 Phylogeny of the Land Snails 249 Age of the Land Mollusca 254 Land Snail Faunas of the Pacific Ocean Area 264 Land Snail Regions of the Indo-Pacific Area 305 converted by Web2PDFConvert.com Origin of the New Hebridean Fauna 311 Discussion 329 Conclusions 331 References 334 241 LIST OF ILLUSTRATIONS TEXT FIGURES PAGE 9. Proportionate representation of land snail orders in different faunas. ... 250 10. Phylogeny of land Mollusca 252 11. Phylogeny of Stylommatophora 253 12. Range of Streptaxidae, Corillidae, Caryodidae, Partulidae, and Assi- mineidae 266 13. Range of Punctinae, "Flammulinidae," and Tornatellinidae 267 14. Range of Clausiliidae, Pupinidae, and Helicinidae 268 15. Range of Bulimulidae, large Helicarionidae, and Microcystinae 269 16. Range of endemic Enidae, Cyclophoridae, Poteriidae, Achatinellidae and Amastridae 270 17. -

Fauna of New Zealand Website Copy 2010, Fnz.Landcareresearch.Co.Nz
aua o ew eaa Ko te Aiaga eeke o Aoeaoa IEEAE SYSEMAICS AISOY GOU EESEAIES O ACAE ESEAC ema acae eseac ico Agicuue & Sciece Cee P O o 9 ico ew eaa K Cosy a M-C aiièe acae eseac Mou Ae eseac Cee iae ag 917 Aucka ew eaa EESEAIE O UIESIIES M Emeso eame o Eomoogy & Aima Ecoogy PO o ico Uiesiy ew eaa EESEAIE O MUSEUMS M ama aua Eiome eame Museum o ew eaa e aa ogaewa O o 7 Weigo ew eaa EESEAIE O OESEAS ISIUIOS awece CSIO iisio o Eomoogy GO o 17 Caea Ciy AC 1 Ausaia SEIES EIO AUA O EW EAA M C ua (ecease ue 199 acae eseac Mou Ae eseac Cee iae ag 917 Aucka ew eaa Fauna of New Zealand Ko te Aitanga Pepeke o Aotearoa Number / Nama 38 Naturalised terrestrial Stylommatophora (Mousca Gasooa Gay M ake acae eseac iae ag 317 amio ew eaa 4 Maaaki Whenua Ρ Ε S S ico Caeuy ew eaa 1999 Coyig © acae eseac ew eaa 1999 o a o is wok coee y coyig may e eouce o coie i ay om o y ay meas (gaic eecoic o mecaica icuig oocoyig ecoig aig iomaio eiea sysems o oewise wiou e wie emissio o e uise Caaoguig i uicaio AKE G Μ (Gay Micae 195— auase eesia Syommaooa (Mousca Gasooa / G Μ ake — ico Caeuy Maaaki Weua ess 1999 (aua o ew eaa ISS 111-533 ; o 3 IS -7-93-5 I ie 11 Seies UC 593(931 eae o uIicaio y e seies eio (a comee y eo Cosy usig comue-ase e ocessig ayou scaig a iig a acae eseac M Ae eseac Cee iae ag 917 Aucka ew eaa Māoi summay e y aco uaau Cosuas Weigo uise y Maaaki Weua ess acae eseac O o ico Caeuy Wesie //wwwmwessco/ ie y G i Weigo o coe eoceas eicuaum (ue a eigo oaa (owe (IIusao G M ake oucio o e coou Iaes was ue y e ew eaIa oey oa ue oeies eseac -

A Phylogeny of the Cannibal Snails of Southern Africa, Genus Natalina Sensu Lato (Pulmonata: Rhytididae): Assessing Concordance Between Morphology and Molecular Data
Molecular Phylogenetics and Evolution 52 (2009) 167–182 Contents lists available at ScienceDirect Molecular Phylogenetics and Evolution journal homepage: www.elsevier.com/locate/ympev A phylogeny of the cannibal snails of southern Africa, genus Natalina sensu lato (Pulmonata: Rhytididae): Assessing concordance between morphology and molecular data Adnan Moussalli a,b,c,*, David G. Herbert a,b, Devi Stuart-Fox d a School of Biological and Conservation Sciences, University of KwaZulu-Natal, Pietermaritzburg 3206, South Africa b Department of Mollusca, Natal Museum, P. Bag 9070, Pietermaritzburg 3200, South Africa c Sciences Department, Museum Victoria, Carlton, Vic. 3053, Australia d Department of Zoology, University of Melbourne, Melbourne, Vic. 3010, Australia article info abstract Article history: The genus Natalina Pilsbry, 1893 is a southern African endemic belonging to the Gondwanan family of Received 12 October 2008 carnivorous snails, Rhytididae. We present a well-resolved molecular phylogeny of the genus based on Revised 14 January 2009 the mitochondrial 16S and COI genes and the nuclear ITS2 gene, and assess this in light of Watson’s [Wat- Accepted 20 February 2009 son, H., 1934. Natalina and other South African snails. Proc. Malacol. Soc. Lond. 21, 150–193] supra-spe- Available online 1 March 2009 cific classification via a re-examination of 23 morphological characters including features of the shell, radula, external anatomy and distal reproductive tract. Ancestral reconstruction and character mapping Keywords: based on the MK model reveals broad concordance between morphology and the molecular phylogeny Natalina 1 at the supra-specific level. Given this concordance and exceptionally deep divergences in the molecular Rhytididae Mitochondrial data, we recommend the elevation of the subgenera Natalina s.s., Afrorhytida, and Capitina to generic sta- Nuclear tus. -
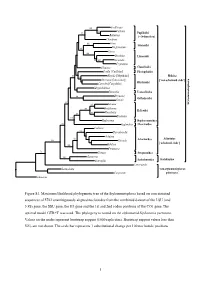
Figure S1. Maximum Likelihood Phylogenetic Tree of The
100 Cochlicopa 55 Vallonia 92 Pupilloidei Buliminus [= Orthurethra] Chondrina Arion 100 Arionoidei 66 Meghimatium Vitrina 100 Oxychilus Limacoidei 82 100 Euconulus Cryptozona Albinaria Clausilioidei Corilla [Corillidae] Plectopyloidea 70 Rhytida [Rhytididae] Helicina 53 Dorcasia [Dorcasiidae] [‘non-achatinoid clade’] Caryodes [Caryodidae] Rhytidoidei Megalobulimus Testacella Testacelloidea Drymaeus 94 Orthalicoidei Gaeotis 82 93 Satsuma Stylommatophora 100 Bradybaena Helicoidei Monadenia 87 93 84 Trochulus Haplotrema Haplotrematoidea 93 Euglandina Oleacinoidea Coeliaxis 92 Thyrophorella Achatina 92 Achatinina 100 Glessula Achatinoidea [‘achatinoid clade’] 100 Subulina Ferussacia 76 Gonaxis Streptaxoidea 100 Guestieria Systrophia Scolodontoidea Scolodontina Laevicaaulis Laemodonta ‘non-stylommatophoran Carychium pulmonates’ Siphonaria 1% 0.01 Figure S1. Maximum likelihood phylogenetic tree of the Stylommatophora based on concatenated sequences of 5782 unambiguously aligned nucleotides from the combined dataset of the LSU (and 5.8S) gene, the SSU gene, the H3 gene and the 1st and 2nd codon positions of the CO1 gene. The optimal model GTR+G was used. The phylogeny is rooted on the siphonariid Siphonaria pectinata. Values on the nodes represent bootstrap support (1000 replicates). Bootstrap support values less than 50% are not shown. The scale bar represents 1 substitutional change per 100 nucleotide positions. 1 91 Satsuma 100 Bradybaena Trochulus 97 Helicoidei 68 Monadenia 87 Haplotrema Haplotrematoidea Euglandina Oleacinoidea 100 Vallonia -

Distribution and Status of Native Carnivorous Land Snails in The
5. Results 5.1 SUMMARY OF SPECIES DISTRIBUTIONS The distribution database contains 1187 locality records, 927 for Rhytida and 260 for Wainuia, including some duplicates. Six percent of all records are of subfossils, mostly from cave deposits. Fig. 2 shows the combined occurrence of all species by 10 000 m x 10 000 m grid square. At least one species was recorded from 406 grid squares (391 excluding subfossil records). Rhytida species were recorded in 333 squares (318 excluding subfossils) and Wainuia species in 94 squares (90 excluding subfossils). The number of 10 000 m x 10 000 m grid squares containing records of each species or subspecies ranged from 2 to 105 (Table 1). Subfossil records extended the known range to additional grid squares in 12 of the 17 taxa. Generalised range maps for all taxa are presented for quick reference in Fig. 3. The distributions are mapped in detail in Fig. 4 (see Appendix 4) and discussed in the species accounts (Appendix 4). 5.2 ASSESSMENT OF CONSERVATION STATUS Data relevant to conservation status are discussed in the species notes (Appendix 4). Direct assessment of population trends is not currently possible as no population study has been conducted for any species of Wainuia or Rhytida. A tentative priority ranking was therefore performed using the scoring system adopted for other invertebrates in Department of Conservation (1994b). The results provide a coarse and somewhat subjective indication of conservation priorities (Table 2). They can be criticised for failing to adequately incorporate uncertainty in our present knowledge, and for not identifying critically threatened populations within some species or subspecies. -

Distribution and Status of Native Carnivorous Land Snails in the Genera Wainuia and Rhytida
Distribution and status of native carnivorous land snails in the genera Wainuia and Rhytida SCIENCE FOR CONSERVATION: 101 Murray Efford Published by Department of Conservation P.O. Box 10-420 Wellington, New Zealand Science for Conservation presents the results of investigations by DOC staff, and by contracted science providers outside the Department of Conservation. Publications in this series are internally and externally peer reviewed. © December 1998, Department of Conservation ISSN 11732946 ISBN 0478217714 This publication originated from work done under Department of Conservation Investigation no. 790, carried out by Murray Efford, Manaaki Whenua - Landcare Research, Private Bag 1930, Dunedin. It was approved for publication by the Director, Science & Research Unit, Science Technology and Information Services, Department of Conservation, Wellington. Cataloguing in Publication Efford, M. G. Distribution and status of native carnivorous land snails in the genera Wainuia and Rhytida / Murray Efford. Wellington, N.Z. : Dept. of Conservation, 1998. 1 v. ; 30 cm. (Science for conservation, 11732946 ; 101.) Includes bibliographical references. ISBN 0478217714 1. SnailsNew Zealand. 2. Rhytididae. I. Title. II. Series: Science for conservation (Wellington, N.Z.) ; 101. 594.30993 20 zbn98112337 CONTENTS Abstract 5 1. Introduction 5 2. Background 5 2.1 Systematics 9 Species-level taxonomy 9 2.2 Previous research on Wainuia and Rhytida 12 2.3 Conservation status 12 3. Objectives 15 4. Methods 13 5. Results 14 5.1 Summary of species distributions 14 5.2 Assessment of conservation status 14 6. Discussion 14 6.1 Distribution 14 6.2 Conservation 18 General policy 18 Vulnerable populations 18 General management needs 19 7. Recommendations 20 8. -

Wildlife Amendment
[As REPORTED FROM THE LANDS AND ARICULTURE COMMITTEE] House of Representatives,23 May 1980. Words sfruck out are shown in italics within bold round brackets, or with black rule at beginning and after last line; words inserted are shown in roman underlined with a double rule, or with double rule before first line and after last line. Hon. Mr Highet WILDLIFE AMENDMENT ANALYSIS Wildlife, Management Reserves 14A. Wildlife management re- serves Management Plans Title 1. Short Title 148. Management plans 2. Interpretation 14c. Review of management 3. Terrestrial and freshwater inverte- plans brates 7. MinisteFs general powers 4. Pollution of wildlife sanctuaries 8. Delegation of Minister's powers 5. Wildlife refuges 9. Increase in penalties for offences 6. Wildlife management reserves and 10. Evidence of boundaries, etc. management plans Schedules A BILL INTITULED An Act to amend the Wildlife Act 1953 BE IT ENACTED by the General Assembly of New Zealand, in Parliament assembled, and by the authority of the same, 5 as follows: 1. Short Title-This Act may be cited as the Wildlife Amendment Act C /979) 1980, and shall be read together with and deemed part of the Wildlife Act 1953* (herein- after referred to as the principal Act). 10 2. Interpretation- (1) Section 2 of the principal Act is hereby amended by inserting, in the definition of the term "animal" after the word "amphibian", the words "; and *1957 Reprint, Vol. 16, p. 669 Amendments: 1959, No. 49; 1964, No. 63; 1966, No. 94; 1968, No. 120; 1971, No. 140; 1972, No. 116; 1973, No. 99 No. -

Supplementary Material (257.14
Veale et al.: Te reo Ma-ori in taxonomy S1 Supplementary Materials Arthripoda Crustacea Appendix S1. List of taxa recorded with te reo / ta re epithets. Decapoda crabs Species Antipodarctus aoteanus, Powell 1949 Animals Ctenocheles maorianus, Powell 1949 Gandalfus puia, McLay 2007 Protostomia Halimena aotearoa, Melrose 1975 Gordioidea Gordian worms Homolodromia kai, Guinot 1993 Gordionus maori, Yadav & Tobias & Schmidt-Rhaesa 2018 Lebbeus wera, Ahyong 2009 Lithodes aotearoa, Ahyong 2010 Nemetea Munidopsis maunga, Schnabel & Bruce 2006 Anopla Nemertean worms Notosceles pepeke, Dawson & Yaldwyn 2000 Praealbonemertes whangateaunienses, Cantell 2012 Platymaia maoria, Dell 1963 Rhynchocinetes ikatere, Yaldwyn 1971 Nematodea Uroptychus kaitara, Schnabel 2009 Longidorus waikouaitii, Yeates & Boag & Brown 1997 Uroptychus maori, Borradaile 1916 Procyrnea kea, Clark 1978 Uroptychus paku, Schnabel 2009 Sciadiocara tarapunga, Clark 1978 Uroptychus rutua, Schnabel 2009 Takamangai waenga, Yeates 1967 Uroptychus toka, Schnabel 2009 Viktorocara torea, Clark 1978 Xenograpsus ngatama, McLay 2007 Onychophora Velvet worms Amphipoda Amphipods Peripatoides kawekaensis, Trewick 1998 Genera Kanikania, Duncan 1994 Annelida worms Makawe, Duncan 1994 Genera Ringanui, Fenwick 2006 Maoridrilus, Michaelsen 1899 Puhuruhuru, Duncan 1994 Tara, Duncan 1994 Species Waematu, Duncan 1994 Amborhytida tarangaensis, Powell 1930 Aporodrilus aotea, Blakemore 2011 Species Aporodtrilus ponga, Blackmore 2011 Chiltonia mihiwaka, Chilton 1898 Cytora aranea, Powell 1928 Kanikania motuensis, -

Biodiversidad Y Endemismo De Los Caracoles Terrestres Megalobulimus Y Systrophia En La Amazonia Occidental
Rev. peru. biol. 19(1): 059 - 074 (Abril 2012) © Facultad de Ciencias Biológicas UNMSM Biodiversidad y endemismo de MegalobulimusISSN y Systrophia1561-0837 Biodiversidad y endemismo de los caracoles terrestres Megalobulimus y Systrophia en la Amazonia occidental Biodiversity and endemism of the western Amazonia land snails Megalobulimus and Systrophia Rina Ramírez 1, 2, Víctor Borda 1, 2, Pedro Romero 1, 2, Jorge Ramirez 1, 2, Carlos Congrains 1, 2, Jenny Chirinos 1, 2, Pablo Ramírez 3, Luz Elena Velásquez 4, Kember Mejía 5 Resumen 1 Museo de Historia Natural, Uni- versidad Nacional Mayor de San En este trabajo realizamos un estudio biogeográfico de dos géneros de caracoles terrestres amazónicos, Marcos. Apartado 14-0434, Lima- 14, Perú. Megalobulimus (Strophocheilidae) y Systrophia (Scolodontidae). Se utilizaron individuos colectados en diversas 2 Laboratorio de Sistemática Mole- localidades de la Amazonia peruana así como información bibliográfica. Se utilizaron los marcadores molecu- cular y Filogeografía, Facultad de lares 5.8S-ITS2-28S rRNA y 16S rRNA para reconstruir filogenias y obtener hipótesis sobre las relaciones Ciencias Biológicas, Universidad evolutivas entre los géneros amazónicos y otras especies de distribución global. La filogenia nuclear permitió Nacional Mayor de San Marcos, Av. Venezuela s/n, Lima-1, Perú. determinar la posición evolutiva de ambos géneros y la filogenia mitocondrial permitió la diferenciación de las especies a nivel intragenérico. Megalobulimus formó parte del clado no-achatinoideo en la filogenia de 3 Laboratorio de Microbiología Molecular y Biotecnología, Facultad los gastrópodos Stylommatophora, como lo esperado, pero no pudo ser demostrada su cercanía a la familia de Ciencias Biológicas, Universidad Acavidae, mientras que Systrophia quedó fuera de los dos clados establecidos, formando uno basal dentro Nacional Mayor de San Marcos.