Monitoring Strategies for the Giant Land Snail Powelliphanta Traversi
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Internal Correspondence
Internal Correspondence Our ref: Your ref: To: PRSG – T. Wilkes Date: From: Terrestrial Ecosystems Unit – J. Marshall Subject: Te Kuha Coal Mine Summary • The applicant has provided appropriate and adequate information to assess the vegetation and flora values of the proposed industrial footprint, the impact of the proposal on those values and potential mitigation and compensation actions • The vegetation and flora values within the Westport Water Conservation Reserve, the Ballarat and Mount Rochfort Conservation Areas and the Lower Buller Gorge Scenic Reserve are clearly significant, particularly the degree of intactness but also the degree of connectivity to other large and relatively unmodified areas of high ecological value, and because of the presence of several “Naturally Uncommon Ecosystems”, two Nationally Threatened plant species, one and potentially two or three plant species in decline – at risk of extinction, and six species with scientifically interesting distributions. • The site is an ecologically important part of the Ecological District and Region. The elevated Brunner coal measures ecosystems are nationally unique: Te Kuha and Mt William are distinguished from all other parts of the elevated Brunner coal measures as they are the only discrete parts of the system that are essentially intact with no significant disruption to ecological patterns and processes and they represent the best example of coastal hillslope forest remaining on elevated Brunner coal measures. • The impacts, both in short and long time frames on significant biodiversity values of an opencast coal mine and associated infrastructure, are significant; the remedial effects of active restoration and site rehabilitation will be limited. • The suggested mitigation actions include avoidance measures, remedial actions and some mitigation and/or compensation suggestions. -

Summary of Native Bat, Reptile, Amphibian and Terrestrial Invertebrate Translocations in New Zealand
Summary of native bat, reptile, amphibian and terrestrial invertebrate translocations in New Zealand SCIENCE FOR CONSERVATION 303 Summary of native bat, reptile, amphibian and terrestrial invertebrate translocations in New Zealand G.H. Sherley, I.A.N. Stringer and G.R. Parrish SCIENCE FOR CONSERVATION 303 Published by Publishing Team Department of Conservation PO Box 10420, The Terrace Wellington 6143, New Zealand Cover: Male Mercury Islands tusked weta, Motuweta isolata. Originally found on Atiu or Middle Island in the Mercury Islands, these were translocated onto six other nearby islands after being bred in captivity. Photo: Ian Stringer. Science for Conservation is a scientific monograph series presenting research funded by New Zealand Department of Conservation (DOC). Manuscripts are internally and externally peer-reviewed; resulting publications are considered part of the formal international scientific literature. Individual copies are printed, and are also available from the departmental website in pdf form. Titles are listed in our catalogue on the website, refer www.doc.govt.nz under Publications, then Science & technical. © Copyright April 2010, New Zealand Department of Conservation ISSN 1173–2946 (hardcopy) ISSN 1177–9241 (PDF) ISBN 978–0–478–14771–1 (hardcopy) ISBN 978–0–478–14772–8 (PDF) This report was prepared for publication by the Publishing Team; editing by Amanda Todd and layout by Hannah Soult. Publication was approved by the General Manager, Research and Development Group, Department of Conservation, Wellington, New Zealand. In the interest of forest conservation, we support paperless electronic publishing. When printing, recycled paper is used wherever possible. CONTENTS Abstract 5 1. Introduction 6 2. Methods 7 3. -

Copy 3 of Janszoon Toolboxes
Invertebrates in the Abel Tasman National Park The terrestrial biodiversity in the Abel Tasman National Park is awesome! Listed below are some of the species commonly found in the park. See the species identification pages to find out more about each one. Also look for the instructions on how to attract invertebrates into your garden with wētā motels and wooden disks. Invertebrates About 97% of all known animals are invertebrates! Invertebrates are animals without a backbone/spine and are the most varied group of animals on the planet. Most invertebrates in New Zealand are found nowhere else in the world and there are thousands of invertebrates species in New Zealand. Invertebrates are an important part of ecosystems to help to keep the balance in nature in numerous ways. They are responsible for pollinating plants, recycling nutrientsSOCIAL and MEDIA keeping population of other living things stable. Some break FOLLOWERSdown pollutants, build and maintain soils and deal with natural waste. Invertebrates are an important food source for many animals such asVISITORS native birds, frogs, lizards, fish and bats. Some invertebrates also eat other invertebrates (e.g. dragonflies eat mosquitoes). Without invertebratesVENDORS ecosystems AND could not survive: they are essential for a healthy environmentEXHIBITORS . In the Abel Tasman National ParkMEDIA we see ATENDEES invertebrates such as: wētā, snails - powelliphanta hochstetteri & rhytida oconnori, giant earthworm, leaf-veined slug & cicadas. Image: Experiencing invertebrates in your green spaces, p26, Department of Conservation, te papa atawhai Rhytida oconnori Habitat New Zealand rhytida snails typically live under fern or leaf litter and in damp rock piles in unmodified native forest, and under tussock or scrub in the subalpine zone. -

Husbandry of the Carnivorous Land Snail, Powelliphanta Augusta (Gastropoda: Pulmonata: Rhytdidae)
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by ResearchArchive at Victoria University of Wellington Husbandry of the Carnivorous Land Snail, Powelliphanta augusta (Gastropoda: Pulmonata: Rhytdidae) By Thomas Edward Allan A thesis submitted to the Victoria University of Wellington in fulfillment of the requirements for the degree of Master of Science in Ecological Restoration Victoria University of Wellington 2010 1 Abstract Key aspects of the captive husbandry of Powelliphanta augusta, a newly-described New Zealand land snail are investigated: how they should be managed and fed to provide individuals for release, and how a long-term captive population can be maintained as an insurance against extinction in the wild. This project arises from almost all members of this species having been brought into captivity due to their displacement in the wild by an opencast coalmine. Powelliphanta (F: Rhytididae) is a genus of endemic carnivorous snails, which includes 10 species, 27 subspecies and numerous undescribed taxa. As well as its diversity, Powelliphanta is renowned for the large size of its members (up to 90mm diameter) and their attractively-patterned shells. Most taxa are threatened due to habitat loss and predation by introduced mammalian predators. The study commences with a literature review to refine husbandry methods and to assess requirements for captive breeding of snails. From this review investigations are made into stocking densities, substrate, reproductive biology, body condition and growth of the P. augusta captive population. To determine an appropriate stocking density for P. augusta groups of six snails were kept at two densities; with either 720cm2, or 1440cm2 per group. -

Evaluating the Effects of Biogeography and Fragmentation on The
Evaluating the effects of biogeography and fragmentation on the taxonomic, functional, and genetic diversity of forest-utilising bats in a South African biodiversity hotspot by Monika Ilka Moir Dissertation presented for the degree of Doctor of Philosophy in the Faculty of Science at Stellenbosch University Supervisors: Dr. Ramugondo V. Rambau Prof. Michael I. Cherry Dr. Leigh R. Richards December 2020 i Stellenbosch University https://scholar.sun.ac.za Declaration By submitting this dissertation electronically, I declare that the entirety of the work contained therein is my own, original work, that I am the sole author thereof (save to the extent explicitly otherwise stated), that reproduction and publication thereof by Stellenbosch University will not infringe any third party rights, and that I have not previously in its entirety or in part submitted it for obtaining any qualification. This dissertation includes one original paper published in a peer-reviewed journal with me as lead author, and three articles submitted and under peer-review. The development and writing of the papers (published and unpublished) were the principal responsibility of myself. Monika Ilka Moir August 2020 Copyright © 2020 Stellenbosch University All rights reserved ii Stellenbosch University https://scholar.sun.ac.za Abstract Bats are a highly diverse mammalian order and are some of the most economically important non-domesticated vertebrates, providing many ecosystem services that contribute to the global economy. Yet, they remain a largely understudied taxon, particularly in the Eastern Cape province of South Africa, in which basic surveys of bat assemblages utilising indigenous forests are lacking. Indigenous forests constitute South Africa’s smallest and most fragmented biome yet support disproportionally high biodiversity. -

Fauna of New Zealand Website Copy 2010, Fnz.Landcareresearch.Co.Nz
aua o ew eaa Ko te Aiaga eeke o Aoeaoa IEEAE SYSEMAICS AISOY GOU EESEAIES O ACAE ESEAC ema acae eseac ico Agicuue & Sciece Cee P O o 9 ico ew eaa K Cosy a M-C aiièe acae eseac Mou Ae eseac Cee iae ag 917 Aucka ew eaa EESEAIE O UIESIIES M Emeso eame o Eomoogy & Aima Ecoogy PO o ico Uiesiy ew eaa EESEAIE O MUSEUMS M ama aua Eiome eame Museum o ew eaa e aa ogaewa O o 7 Weigo ew eaa EESEAIE O OESEAS ISIUIOS awece CSIO iisio o Eomoogy GO o 17 Caea Ciy AC 1 Ausaia SEIES EIO AUA O EW EAA M C ua (ecease ue 199 acae eseac Mou Ae eseac Cee iae ag 917 Aucka ew eaa Fauna of New Zealand Ko te Aitanga Pepeke o Aotearoa Number / Nama 38 Naturalised terrestrial Stylommatophora (Mousca Gasooa Gay M ake acae eseac iae ag 317 amio ew eaa 4 Maaaki Whenua Ρ Ε S S ico Caeuy ew eaa 1999 Coyig © acae eseac ew eaa 1999 o a o is wok coee y coyig may e eouce o coie i ay om o y ay meas (gaic eecoic o mecaica icuig oocoyig ecoig aig iomaio eiea sysems o oewise wiou e wie emissio o e uise Caaoguig i uicaio AKE G Μ (Gay Micae 195— auase eesia Syommaooa (Mousca Gasooa / G Μ ake — ico Caeuy Maaaki Weua ess 1999 (aua o ew eaa ISS 111-533 ; o 3 IS -7-93-5 I ie 11 Seies UC 593(931 eae o uIicaio y e seies eio (a comee y eo Cosy usig comue-ase e ocessig ayou scaig a iig a acae eseac M Ae eseac Cee iae ag 917 Aucka ew eaa Māoi summay e y aco uaau Cosuas Weigo uise y Maaaki Weua ess acae eseac O o ico Caeuy Wesie //wwwmwessco/ ie y G i Weigo o coe eoceas eicuaum (ue a eigo oaa (owe (IIusao G M ake oucio o e coou Iaes was ue y e ew eaIa oey oa ue oeies eseac -

Notification of Access Arrangement for MP 41279, Mt Te Kuha
Attachment C Draft Terrestrial Ecology Report 106 VEGETATION AND FAUNA OF THE PROPOSED TE KUHA MINE SITE Prepared for Te Kuha Limited Partnership October 2013 EXECUTIVE SUMMARY The Te Kuha mining permit is located predominantly within the Westport Water Conservation Reserve (1,825 ha), which is a local purpose reserve administered by the Buller District Council. The coal deposit is situated outside the water catchment within an area of approximately 490 ha of Brunner Coal Measures vegetation approximately 5 km southwest of Mt Rochfort. Access would be required across conservation land to reach the coal resource. The Te Kuha site was recommended as an area for protection by the Protected Natural Areas Programme surveys in the 1990s on the basis that in the event it was removed from the local purpose reserve for any reason, addition to the public conservation estate would increase the level of protection of coal measures habitats which, although found elsewhere (principally in the Mt Rochfort Conservation Area), were considered inadequately protected overall. The proposal to create an access road and an opencast mine at the site would affect twelve different vegetation types to varying degrees. The habitats present at the proposed mine site are overwhelmingly indigenous and have a very high degree of intactness reflecting their lack of human disturbance. Previous surveys have shown that some trees in the area are more than 500 years old. Habitats affected by the proposed access road are less intact and include exotic pasture as well as regenerating shrubland and forest. Te Kuha is not part of the Department of Conservation’s Buller Coal Plateaux priority site and is unlikely to receive management for that reason. -

New Zealand's Threatened Species Strategy
NEW ZEALAND’S THREATENED SPECIES STRATEGY DRAFT FOR CONSULTATION Toitū te marae a Tāne-Mahuta, Toitū te marae a Tangaroa, Toitū te tangata. If the land is well and the sea is well, the people will thrive. From the Minister ew Zealand’s unique While Predator Free 2050 is the single most significant and Nplants, birds, reptiles ambitious conservation programme in our history, it has to and other animal species be part of a broader range of work if we are to succeed. help us to define who we This draft Threatened Species Strategy is the are as a nation. Familiar Government’s plan to halt decline and restore healthy, emblems include our sustainable populations of native species. The Strategy flightless nocturnal kiwi looks at what steps are needed to restore those species and kākāpō, and the at risk of extinction, and what we should do to prevent silver fern proudly worn others from becoming threatened. by our sportspeople and etched on our war graves We are deliberately using the language of war because we and memorials. are up against invasive enemies that are hard to defeat. If we are to save the creatures we love, we have to eradicate They are our national the predators intent on eating them to extinction. taonga, living treasures found nowhere else on Earth – the unique creations of In response to beech tree seeding ‘mast’ years we have millions of years of geographical isolation. launched the successful Battle for our Birds – pest control on a landscape scale. We have declared a War on Weeds The wildlife on our islands of Aotearoa evolved in a with an annual list of the ‘Dirty Dozen’ to tackle invasive world without teeth, a paradise which for all its stunning plants that are suffocating vast areas of our bush. -

A Phylogeny of the Cannibal Snails of Southern Africa, Genus Natalina Sensu Lato (Pulmonata: Rhytididae): Assessing Concordance Between Morphology and Molecular Data
Molecular Phylogenetics and Evolution 52 (2009) 167–182 Contents lists available at ScienceDirect Molecular Phylogenetics and Evolution journal homepage: www.elsevier.com/locate/ympev A phylogeny of the cannibal snails of southern Africa, genus Natalina sensu lato (Pulmonata: Rhytididae): Assessing concordance between morphology and molecular data Adnan Moussalli a,b,c,*, David G. Herbert a,b, Devi Stuart-Fox d a School of Biological and Conservation Sciences, University of KwaZulu-Natal, Pietermaritzburg 3206, South Africa b Department of Mollusca, Natal Museum, P. Bag 9070, Pietermaritzburg 3200, South Africa c Sciences Department, Museum Victoria, Carlton, Vic. 3053, Australia d Department of Zoology, University of Melbourne, Melbourne, Vic. 3010, Australia article info abstract Article history: The genus Natalina Pilsbry, 1893 is a southern African endemic belonging to the Gondwanan family of Received 12 October 2008 carnivorous snails, Rhytididae. We present a well-resolved molecular phylogeny of the genus based on Revised 14 January 2009 the mitochondrial 16S and COI genes and the nuclear ITS2 gene, and assess this in light of Watson’s [Wat- Accepted 20 February 2009 son, H., 1934. Natalina and other South African snails. Proc. Malacol. Soc. Lond. 21, 150–193] supra-spe- Available online 1 March 2009 cific classification via a re-examination of 23 morphological characters including features of the shell, radula, external anatomy and distal reproductive tract. Ancestral reconstruction and character mapping Keywords: based on the MK model reveals broad concordance between morphology and the molecular phylogeny Natalina 1 at the supra-specific level. Given this concordance and exceptionally deep divergences in the molecular Rhytididae Mitochondrial data, we recommend the elevation of the subgenera Natalina s.s., Afrorhytida, and Capitina to generic sta- Nuclear tus. -

A Review of Non-Invasive DNA Sampling M
bioRxiv preprint doi: https://doi.org/10.1101/385120; this version posted July 2, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 1 Blood, sweat and tears: a review of non-invasive DNA sampling 2 M.-C. LEFORT*, Laboratoire d'Écologie et Biologie des Interactions (EBI) – UMR 7267 CNRS, 3 Université de Poitiers, 5 rue Albert Turpain, 86073 POITIERS Cedex 9, France | Environmental 4 and Animal Sciences, Unitec Institute of Technology, 139 Carrington Road, Mt 5 Albert, Auckland 1025, New Zealand. 6 R. H. CRUICKSHANK, Department of Ecology, Faculty of Agricultural and Life Sciences, PO 7 Box 85084, Lincoln University, Lincoln 7647, Christchurch, New Zealand. 8 K. DESCOVICH, Environmental and Animal Sciences, Unitec Institute of Technology, 139 9 Carrington Road, Mt Albert, Auckland 1025, New Zealand. | Centre for Animal Welfare and 10 Ethics, University of Queensland, Gatton 4343, Queensland, Australia 11 N. J. ADAMS, Environmental and Animal Sciences, Unitec Institute of Technology, 139 12 Carrington Road, Mt Albert, Auckland 1025, New Zealand. 13 A. BARUN, Department of Ecology, Faculty of Agricultural and Life Sciences, PO Box 85084, 14 Lincoln University, Lincoln 7647, Christchurch, New Zealand. 15 A. EMAMI-KHOYI, Centre for Ecological Genomics and Wildlife Conservation, University of 16 Johannesburg, Auckland Park 2006, South Africa | Department of Ecology, Faculty of 17 Agricultural and Life Sciences, PO Box 85084, Lincoln University, Lincoln 7647, Christchurch, 18 New Zealand. -

Hon Eugenie Sage
Hon Eugenie Sage Minister of Conservation Minita mō Te Papa Atawhai EMBARGOED UNTIL 08:00 13 March 2019 PĀNUI PĀPĀHO MEDIA STATEMENT Mokihinui River catchment land to be added to Kahurangi National Park Conservation Minister Eugenie Sage today announced the largest addition of land to an existing national park in New Zealand’s history. A total of 64,400 hectares of conservation land in the Mokihinui River catchment on the West Coast north of Westport, including 15 km of riverbed, is being added to Kahurangi National Park. “Adding this area, roughly half the size of Auckland City, to Kahurangi is the largest addition of land to an existing national park in New Zealand’s history,” Eugenie Sage said. “National park status will ensure stronger protection of the Mokihinui area’s significant cultural, ecological, historic and recreational values. “A hydro-electric dam was proposed for the Mokihinui River in 2007. The hydro scheme attracted considerable public interest and strong opposition because of its environmental impacts. It would have flooded the Mokihinui Gorge and inundated beech-podocarp forests and significant habitats of threatened plants and wildlife such as whio/blue duck, kaka, bats and giant land snails. “A big thanks to the many New Zealanders and the Department of Conservation who spoke up for the river, its gorge, dramatic landscapes, beech-podocarp forests and set out the reasons they deserved protection from a hydro dam. “Today’s announcement is only possible because of that work and advocacy. It is why our Government can now give the Mokihinui Gorge, and the surrounding lands, forests, and mountains the strong protection that comes with being part of a national park. -
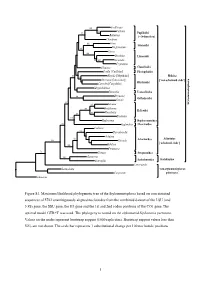
Figure S1. Maximum Likelihood Phylogenetic Tree of The
100 Cochlicopa 55 Vallonia 92 Pupilloidei Buliminus [= Orthurethra] Chondrina Arion 100 Arionoidei 66 Meghimatium Vitrina 100 Oxychilus Limacoidei 82 100 Euconulus Cryptozona Albinaria Clausilioidei Corilla [Corillidae] Plectopyloidea 70 Rhytida [Rhytididae] Helicina 53 Dorcasia [Dorcasiidae] [‘non-achatinoid clade’] Caryodes [Caryodidae] Rhytidoidei Megalobulimus Testacella Testacelloidea Drymaeus 94 Orthalicoidei Gaeotis 82 93 Satsuma Stylommatophora 100 Bradybaena Helicoidei Monadenia 87 93 84 Trochulus Haplotrema Haplotrematoidea 93 Euglandina Oleacinoidea Coeliaxis 92 Thyrophorella Achatina 92 Achatinina 100 Glessula Achatinoidea [‘achatinoid clade’] 100 Subulina Ferussacia 76 Gonaxis Streptaxoidea 100 Guestieria Systrophia Scolodontoidea Scolodontina Laevicaaulis Laemodonta ‘non-stylommatophoran Carychium pulmonates’ Siphonaria 1% 0.01 Figure S1. Maximum likelihood phylogenetic tree of the Stylommatophora based on concatenated sequences of 5782 unambiguously aligned nucleotides from the combined dataset of the LSU (and 5.8S) gene, the SSU gene, the H3 gene and the 1st and 2nd codon positions of the CO1 gene. The optimal model GTR+G was used. The phylogeny is rooted on the siphonariid Siphonaria pectinata. Values on the nodes represent bootstrap support (1000 replicates). Bootstrap support values less than 50% are not shown. The scale bar represents 1 substitutional change per 100 nucleotide positions. 1 91 Satsuma 100 Bradybaena Trochulus 97 Helicoidei 68 Monadenia 87 Haplotrema Haplotrematoidea Euglandina Oleacinoidea 100 Vallonia