Evaluating the Effects of Biogeography and Fragmentation on The
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Filogeografía De Systrophia Helicycloides: El Reflejo De La Dinámica Del Bosque Lluvioso Tropical En Los Genes 16S Rrna Y COI De Moluscos Terrestres
UNIVERSIDAD NACIONAL MAYOR DE SAN MARCOS FACULTAD DE CIENCIAS BIOLÓGICAS UNIDAD DE POSTGRADO Filogeografía de Systrophia helicycloides: el reflejo de la dinámica del bosque lluvioso tropical en los genes 16S rRNA y COI de moluscos terrestres TESIS para optar el grado académico de Magíster en Biología Molecular AUTOR Pedro Eduardo Romero Condori ASESORA Rina L. Ramírez Mesias Lima – Perú 2010 "In the end, we will conserve only what we love; we will love only what we understand; and we will understand only what we are taught." (Baba Dioum, 1968) ii Para Katherine ¡buenas salenas! iii AGRADECIMIENTOS A Emma, por continuar apoyándome en esta aventura, día tras día, y a Pedro por acompañarme ahora desde un buen lugar, para toda la vida. A la Dra. Rina Ramírez, por enseñarme a buscar las preguntas, y encontrar las respuestas a este “misterio de los misterios”, tal como llamaba Charles Darwin al origen de las especies. Es una suerte tenerla como referente académico, y más aún como ejemplo de vida. Este trabajo pudo ser realizado gracias al apoyo de la Universidad Nacional Mayor de San Marcos a través del Vicerrectorado de Investigación y el Instituto de Ciencias Biológicas Antonio Raimondi que aprobaron los proyectos: “Evaluación de la biodiversidad de moluscos en la región del río Bajo Madre de Dios”, “Diversidad genética en la Amazonia: Polimorfismo del genoma mitocondrial de moluscos terrestres de la familia Systrophiidae” y “Biodiversidad de la Familia Systrophiidae (Mollusca, Gastropoda) en la cuenca del Río Los Amigos (Dpto. Madre de Dios). Al Concejo Nacional de Ciencia y Tecnología (CONCYTEC), por la beca para finalizar mis estudios de maestría, fue un honor ser elegido en este grupo de jóvenes profesionales peruanos ganadores de una beca de posgrado en 2008, esta tesis es un sincero esfuerzo para retribuir la confianza y el apoyo prestado. -

South Africa 3Rd to 22Nd September 2015 (20 Days)
Hollyhead & Savage Trip Report South Africa 3rd to 22nd September 2015 (20 days) Female Cheetah with cubs and Impala kill by Heinz Ortmann Trip Report compiled by Tour Leader: Heinz Ortmann Trip Report Hollyhead & Savage Private South Africa September 2015 2 Tour Summary A fantastic twenty day journey that began in the beautiful Overberg region and fynbos of the Western Cape, included the Wakkerstroom grasslands, coastal dune forest of iSimangaliso Wetland Park, the Baobab-studded hills of Mapungubwe National Park and ended along a stretch of road searching for Kalahari specials north of Pretoria amongst many others. We experienced a wide variety of habitats and incredible birds and mammals. An impressive 400-plus birds and close to 50 mammal species were found on this trip. This, combined with visiting little-known parts of South Africa such as Magoebaskloof and Mapungubwe National Park, made this tour special as well as one with many unforgettable experiences and memories for the participants. Our journey started out from Cape Town International Airport at around lunchtime on a glorious sunny early-spring day. Our journey for the first day took us eastwards through the Overberg region and onto the Agulhas plains where we spent the next two nights. The farmlands in these parts appear largely barren and consist of single crop fields and yet host a surprising number of special, localised and endemic species. Our afternoon’s travels through these parts allowed us views of several more common and widespread species such as Egyptian and Spur-winged Geese, raptors like Jackal Buzzard, Rock Kestrel and Yellow-billed Kite, Speckled Pigeons, Capped Wheatear, Pied Starling, the ever present Pied and Cape Crow, White-necked Raven and Pin-tailed Whydah, almost in full breeding plumage. -

Know Your National Parks
KNOW YOUR NATIONAL PARKS 1 KNOW YOUR NATIONAL PARKS KNOW YOUR NATIONAL PARKS Our Parks, Our Heritage Table of contents Minister’s Foreword 4 CEO’s Foreword 5 Northern Region 8 Marakele National Park 8 Golden Gate Highlands National Park 10 Mapungubwe National Park and World Heritage site 11 Arid Region 12 Augrabies Falls National Park 12 Kgalagadi Transfrontier Park 13 Mokala National Park 14 Namaqua National Park 15 /Ai/Ais-Richtersveld Transfrontier Park 16 Cape Region 18 Table Mountain National Park 18 Bontebok National Park 19 Agulhas National Park 20 West Coast National Park 21 Tankwa-Karoo National Park 22 Frontier Region 23 Addo Elephant National Park 23 Karoo National Park 24 DID YOU Camdeboo National Park 25 KNOW? Mountain Zebra National Park 26 Marakele National Park is Garden Route National Park 27 found in the heart of Waterberg Mountains.The name Marakele Kruger National Park 28 is a Tswana name, which Vision means a ‘place of sanctuary’. A sustainable National Park System connecting society Fun and games 29 About SA National Parks Week 31 Mission To develop, expand, manage and promote a system of sustainable national parks that represent biodiversity and heritage assets, through innovation and best practice for the just and equitable benefit of current and future generation. 2 3 KNOW YOUR NATIONAL PARKS KNOW YOUR NATIONAL PARKS Minister’s Foreword CEO’s Foreword We are blessed to live in a country like ours, which has areas by all should be encouraged through a variety of The staging of SA National Parks Week first took place been hailed as a miracle in respect of our transition to a programmes. -

Husbandry of the Carnivorous Land Snail, Powelliphanta Augusta (Gastropoda: Pulmonata: Rhytdidae)
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by ResearchArchive at Victoria University of Wellington Husbandry of the Carnivorous Land Snail, Powelliphanta augusta (Gastropoda: Pulmonata: Rhytdidae) By Thomas Edward Allan A thesis submitted to the Victoria University of Wellington in fulfillment of the requirements for the degree of Master of Science in Ecological Restoration Victoria University of Wellington 2010 1 Abstract Key aspects of the captive husbandry of Powelliphanta augusta, a newly-described New Zealand land snail are investigated: how they should be managed and fed to provide individuals for release, and how a long-term captive population can be maintained as an insurance against extinction in the wild. This project arises from almost all members of this species having been brought into captivity due to their displacement in the wild by an opencast coalmine. Powelliphanta (F: Rhytididae) is a genus of endemic carnivorous snails, which includes 10 species, 27 subspecies and numerous undescribed taxa. As well as its diversity, Powelliphanta is renowned for the large size of its members (up to 90mm diameter) and their attractively-patterned shells. Most taxa are threatened due to habitat loss and predation by introduced mammalian predators. The study commences with a literature review to refine husbandry methods and to assess requirements for captive breeding of snails. From this review investigations are made into stocking densities, substrate, reproductive biology, body condition and growth of the P. augusta captive population. To determine an appropriate stocking density for P. augusta groups of six snails were kept at two densities; with either 720cm2, or 1440cm2 per group. -

Shakati Private Game Reserve in Malaria-Free Waterberg/Vaalwater -Only 2 Hours from Pretoria
Shakati Private Game Reserve in Malaria-free Waterberg/Vaalwater -only 2 hours from Pretoria Waterberg. There is so much to see and do…. Waterberg is the area of magnificent views, panoramic savannah and bush landscapes, spectacular mountains and cliffs, crystal clear streams and an unbelievable abundance of wild animals, trees and flowers. Game viewing in the Waterberg area is absolutely fantastic and recognised among the best in the country –hence the Waterberg is one of the preferred eco-tourism destination in South Africa. Furthermore Waterberg with its unspoilt nature has been designated as UNESCO “Savannah Biosphere Reserve” –the first in Southern Africa. And Waterberg is MALARIA-free… Marakele National Park Shakati Private Game Reserve is hidden away on the lush banks of the Mokolo river in the deep heart of the untamed Waterberg bushveld paradise. Near Vaalwater and only 2 hours drive from Pretoria. Time spent at Shakati Game Reserve is about getting away from city life, work, traffic and stress. It is about peace and tranquillity, clean fresh air and clear skies with the brightest stars you have probably ever seen. It is about being quiet and listen to the jackal calling at night, to the paradise flycatcher singing in the morning. It is about seeing and walking with the animals, touching the fruits of the bush willow -and wonder about nature. It is about quietly sitting at the water hole watching game and taking life easy Its time to leave the city sounds, the hustle, the bustle and find some place that speaks to you who you really are inside. -

Herpetological Bulletin
The HERPETOLOGICAL BULLETIN Number 80 — Summer 2002 PUBLISHED BY THE BRITISH HERPETOLOGICAL SOCIETY THE HERPETOLOGICAL BULLETIN The Herpetological Bulletin is produced quarterly and publishes, in English, a range of articles concerned with herpetology. These include full-length papers of mostly a semi-technical nature, book reviews, letters from readers, society news, and other items of general herpetological interest. Emphasis is placed on natural history, conservation, captive breeding and husbandry, veterinary and behavioural aspects. Articles reporting the results of experimental research, descriptions of new taxa, or taxonomic revisions should be submitted to The Herpetological Journal (see inside back cover for Editor's address). ISSN 1473-0928 © The British Herpetological Society. All rights reserved. No part of this publication may be reproduced without the permission of the Editor. Printed by Metloc Printers Limited, Old Station Road, Loughton, Essex. Information for contributors 1. Contributions should be submitted in hard copy form (2 copies of manuscript, double-spaced) AND on computer diskette in Windows format only. The Bulletin is typeset directly from the author's diskette, so wherever possible all manuscripts should be prepared using a word-processor. Please indicate word-processing software used, and if possible also include a text-only version of the file. The text should be arranged in the following order: Title; Name(s) of author(s); Address(es) of authors (please indicate corresponding author); Abstract (optional - if included should not exceed 10% of total word length); Text; Acknowledgements; References; Appendices. Footnotes should not be included. Refer to this issue for style and format information. 2. Slides and high resolution scanned images are the preferred form of illustration, although good quality prints are also acceptable. -

Fauna of New Zealand Website Copy 2010, Fnz.Landcareresearch.Co.Nz
aua o ew eaa Ko te Aiaga eeke o Aoeaoa IEEAE SYSEMAICS AISOY GOU EESEAIES O ACAE ESEAC ema acae eseac ico Agicuue & Sciece Cee P O o 9 ico ew eaa K Cosy a M-C aiièe acae eseac Mou Ae eseac Cee iae ag 917 Aucka ew eaa EESEAIE O UIESIIES M Emeso eame o Eomoogy & Aima Ecoogy PO o ico Uiesiy ew eaa EESEAIE O MUSEUMS M ama aua Eiome eame Museum o ew eaa e aa ogaewa O o 7 Weigo ew eaa EESEAIE O OESEAS ISIUIOS awece CSIO iisio o Eomoogy GO o 17 Caea Ciy AC 1 Ausaia SEIES EIO AUA O EW EAA M C ua (ecease ue 199 acae eseac Mou Ae eseac Cee iae ag 917 Aucka ew eaa Fauna of New Zealand Ko te Aitanga Pepeke o Aotearoa Number / Nama 38 Naturalised terrestrial Stylommatophora (Mousca Gasooa Gay M ake acae eseac iae ag 317 amio ew eaa 4 Maaaki Whenua Ρ Ε S S ico Caeuy ew eaa 1999 Coyig © acae eseac ew eaa 1999 o a o is wok coee y coyig may e eouce o coie i ay om o y ay meas (gaic eecoic o mecaica icuig oocoyig ecoig aig iomaio eiea sysems o oewise wiou e wie emissio o e uise Caaoguig i uicaio AKE G Μ (Gay Micae 195— auase eesia Syommaooa (Mousca Gasooa / G Μ ake — ico Caeuy Maaaki Weua ess 1999 (aua o ew eaa ISS 111-533 ; o 3 IS -7-93-5 I ie 11 Seies UC 593(931 eae o uIicaio y e seies eio (a comee y eo Cosy usig comue-ase e ocessig ayou scaig a iig a acae eseac M Ae eseac Cee iae ag 917 Aucka ew eaa Māoi summay e y aco uaau Cosuas Weigo uise y Maaaki Weua ess acae eseac O o ico Caeuy Wesie //wwwmwessco/ ie y G i Weigo o coe eoceas eicuaum (ue a eigo oaa (owe (IIusao G M ake oucio o e coou Iaes was ue y e ew eaIa oey oa ue oeies eseac -

A Phylogeny of the Cannibal Snails of Southern Africa, Genus Natalina Sensu Lato (Pulmonata: Rhytididae): Assessing Concordance Between Morphology and Molecular Data
Molecular Phylogenetics and Evolution 52 (2009) 167–182 Contents lists available at ScienceDirect Molecular Phylogenetics and Evolution journal homepage: www.elsevier.com/locate/ympev A phylogeny of the cannibal snails of southern Africa, genus Natalina sensu lato (Pulmonata: Rhytididae): Assessing concordance between morphology and molecular data Adnan Moussalli a,b,c,*, David G. Herbert a,b, Devi Stuart-Fox d a School of Biological and Conservation Sciences, University of KwaZulu-Natal, Pietermaritzburg 3206, South Africa b Department of Mollusca, Natal Museum, P. Bag 9070, Pietermaritzburg 3200, South Africa c Sciences Department, Museum Victoria, Carlton, Vic. 3053, Australia d Department of Zoology, University of Melbourne, Melbourne, Vic. 3010, Australia article info abstract Article history: The genus Natalina Pilsbry, 1893 is a southern African endemic belonging to the Gondwanan family of Received 12 October 2008 carnivorous snails, Rhytididae. We present a well-resolved molecular phylogeny of the genus based on Revised 14 January 2009 the mitochondrial 16S and COI genes and the nuclear ITS2 gene, and assess this in light of Watson’s [Wat- Accepted 20 February 2009 son, H., 1934. Natalina and other South African snails. Proc. Malacol. Soc. Lond. 21, 150–193] supra-spe- Available online 1 March 2009 cific classification via a re-examination of 23 morphological characters including features of the shell, radula, external anatomy and distal reproductive tract. Ancestral reconstruction and character mapping Keywords: based on the MK model reveals broad concordance between morphology and the molecular phylogeny Natalina 1 at the supra-specific level. Given this concordance and exceptionally deep divergences in the molecular Rhytididae Mitochondrial data, we recommend the elevation of the subgenera Natalina s.s., Afrorhytida, and Capitina to generic sta- Nuclear tus. -

Threatened Ecosystems in South Africa: Descriptions and Maps
Threatened Ecosystems in South Africa: Descriptions and Maps DRAFT May 2009 South African National Biodiversity Institute Department of Environmental Affairs and Tourism Contents List of tables .............................................................................................................................. vii List of figures............................................................................................................................. vii 1 Introduction .......................................................................................................................... 8 2 Criteria for identifying threatened ecosystems............................................................... 10 3 Summary of listed ecosystems ........................................................................................ 12 4 Descriptions and individual maps of threatened ecosystems ...................................... 14 4.1 Explanation of descriptions ........................................................................................................ 14 4.2 Listed threatened ecosystems ................................................................................................... 16 4.2.1 Critically Endangered (CR) ................................................................................................................ 16 1. Atlantis Sand Fynbos (FFd 4) .......................................................................................................................... 16 2. Blesbokspruit Highveld Grassland -

Flight Restrictions Over National Parks and World Heritage Sites
Physical Address: Postal Address: Telephone Number: E-mail Address: Ikhaya Lokundiza Private Bag X 73 +27 11 545 1000 [email protected] Treur Close Halfway House Waterfall Park 1685 Fax Number: Website Address: Bekker Street +27 11 545 1465 www.caa.co.za Midrand FLIGHT RESTRICTIONS OVER NATIONAL PARKS AND WORLD HERITAGE SITES The purpose of the National Environmental Management: Protected Areas Act 57 of 2003 is to “ provide for the protection and conservation of ecologically viable areas representative of South Africa's biological diversity and its natural landscapes and seascapes; for the establishment of a national register of all national, provincial and local protected areas; for the management of those areas in accordance with national norms and standards; for intergovernmental co-operation and public consultation in matters concerning protected areas; for the continued existence, governance and functions of South African National Parks; and for matters in connection therewith ”. Attention is drawn to section 47(1) of the Act which require a clearance of at least 2 500 FT above the highest point in a Special Nature Reserve, National Park or World Heritage Site. Pilots are cautioned that these altitudes might require aircraft to enter controlled airspace and therefore appropriate ATC clearances are to be obtained. Non-compliance to the Act is considered an offence (See Section 89 of the Act). Charts depicting the relevant National Parks and World Heritage Sites, and tables containing the Minimum Flight Altitudes, are provided to assist pilots with the identification, minimum heights to be flown or the avoidance of these areas in toto. -
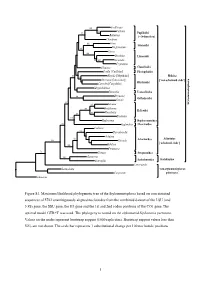
Figure S1. Maximum Likelihood Phylogenetic Tree of The
100 Cochlicopa 55 Vallonia 92 Pupilloidei Buliminus [= Orthurethra] Chondrina Arion 100 Arionoidei 66 Meghimatium Vitrina 100 Oxychilus Limacoidei 82 100 Euconulus Cryptozona Albinaria Clausilioidei Corilla [Corillidae] Plectopyloidea 70 Rhytida [Rhytididae] Helicina 53 Dorcasia [Dorcasiidae] [‘non-achatinoid clade’] Caryodes [Caryodidae] Rhytidoidei Megalobulimus Testacella Testacelloidea Drymaeus 94 Orthalicoidei Gaeotis 82 93 Satsuma Stylommatophora 100 Bradybaena Helicoidei Monadenia 87 93 84 Trochulus Haplotrema Haplotrematoidea 93 Euglandina Oleacinoidea Coeliaxis 92 Thyrophorella Achatina 92 Achatinina 100 Glessula Achatinoidea [‘achatinoid clade’] 100 Subulina Ferussacia 76 Gonaxis Streptaxoidea 100 Guestieria Systrophia Scolodontoidea Scolodontina Laevicaaulis Laemodonta ‘non-stylommatophoran Carychium pulmonates’ Siphonaria 1% 0.01 Figure S1. Maximum likelihood phylogenetic tree of the Stylommatophora based on concatenated sequences of 5782 unambiguously aligned nucleotides from the combined dataset of the LSU (and 5.8S) gene, the SSU gene, the H3 gene and the 1st and 2nd codon positions of the CO1 gene. The optimal model GTR+G was used. The phylogeny is rooted on the siphonariid Siphonaria pectinata. Values on the nodes represent bootstrap support (1000 replicates). Bootstrap support values less than 50% are not shown. The scale bar represents 1 substitutional change per 100 nucleotide positions. 1 91 Satsuma 100 Bradybaena Trochulus 97 Helicoidei 68 Monadenia 87 Haplotrema Haplotrematoidea Euglandina Oleacinoidea 100 Vallonia -

Distribution and Status of Native Carnivorous Land Snails in The
5. Results 5.1 SUMMARY OF SPECIES DISTRIBUTIONS The distribution database contains 1187 locality records, 927 for Rhytida and 260 for Wainuia, including some duplicates. Six percent of all records are of subfossils, mostly from cave deposits. Fig. 2 shows the combined occurrence of all species by 10 000 m x 10 000 m grid square. At least one species was recorded from 406 grid squares (391 excluding subfossil records). Rhytida species were recorded in 333 squares (318 excluding subfossils) and Wainuia species in 94 squares (90 excluding subfossils). The number of 10 000 m x 10 000 m grid squares containing records of each species or subspecies ranged from 2 to 105 (Table 1). Subfossil records extended the known range to additional grid squares in 12 of the 17 taxa. Generalised range maps for all taxa are presented for quick reference in Fig. 3. The distributions are mapped in detail in Fig. 4 (see Appendix 4) and discussed in the species accounts (Appendix 4). 5.2 ASSESSMENT OF CONSERVATION STATUS Data relevant to conservation status are discussed in the species notes (Appendix 4). Direct assessment of population trends is not currently possible as no population study has been conducted for any species of Wainuia or Rhytida. A tentative priority ranking was therefore performed using the scoring system adopted for other invertebrates in Department of Conservation (1994b). The results provide a coarse and somewhat subjective indication of conservation priorities (Table 2). They can be criticised for failing to adequately incorporate uncertainty in our present knowledge, and for not identifying critically threatened populations within some species or subspecies.